Elementary charge
| |||||||||||||||||||||||||||||||||||||||||||||||||

Halaman ini berisi artikel tentang dewa dalam mitologi Yunani. Untuk bulan Jupiter, lihat Io (satelit). Zeus bercinta dengan Io, lukisan karya Antonio da Correggio, 1531 Dalam mitologi Yunani, Io (bahasa Yunani: Ίώ) adalah seorang pendeta Hera di Argos. Dalam mitologi Zeus jatuh cinta pada Io dan menidurinya. Untuk mencegah Hera mengetahui apa yang terjadi, Zeus menutupi dunia dengan awan hitam tebal. Namun Hera malah menjadi curiga, dia lalu turun dari Gunung Olimpus dan menghilangkan awa…

Penyuntingan Artikel oleh pengguna baru atau anonim untuk saat ini tidak diizinkan.Lihat kebijakan pelindungan dan log pelindungan untuk informasi selengkapnya. Jika Anda tidak dapat menyunting Artikel ini dan Anda ingin melakukannya, Anda dapat memohon permintaan penyuntingan, diskusikan perubahan yang ingin dilakukan di halaman pembicaraan, memohon untuk melepaskan pelindungan, masuk, atau buatlah sebuah akun. Untuk kegunaan lain, lihat Dadu (disambiguasi). Dua buah dadu bersisi enam dengan su…
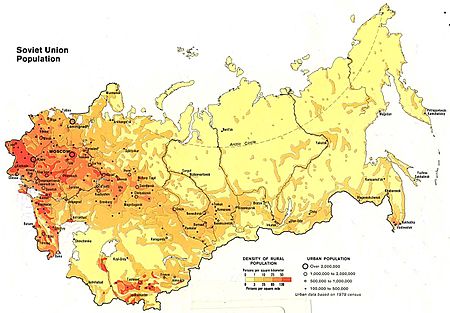
Населе́ние СССР Содержание 1 Численность 2 Демографические показатели 3 Рождаемость 3.1 Рождаемость на 100 жителей РСФСР и младенческая смертность[32] 4 Этнические группы 5 Демографические потери 5.1 Довоенные демографическое потери 5.2 Демографические последствия Великой Отеч…

الدوري المنغولي لكرة القدم 2013 تفاصيل الموسم الدوري المنغولي لكرة القدم البلد منغوليا البطل نادي إركيم عدد المشاركين 7 الدوري المنغولي لكرة القدم 2012 الدوري المنغولي لكرة القدم 2014 تعديل مصدري - تعديل الدوري المنغولي لكرة القدم 2013 هو موسم من الدوري المن…

Orang Yahudi Agama Yahudi Agama Tuhan Allah dalam Yudaisme Dasar Iman Yahudi Kaballah Hari raya Doa Halakha Mitzvot (Daftar: 613) Rabi Sinagoge Pembacaan gulungan Taurat Minhag/Kebiasaan Tzedakah Teks Tanakh: Taurat Nevi'im Ketuvim Literatur Rabinik Talmud Mishnah Gemara Etnis Ashkenazi Sefardim Mizrahi Beta Israel Penduduk (Daftar) Israel AS Rusia/Uni Soviet SpanyolKanada Jerman Prancis Britania Raya Amerika Latin Polandia Dunia Arab Malaysia Yaman Yahudi terkenal menurut negara Daftar Komunita…

British historian and economist The Right HonourableThe Lord SkidelskyFBAOfficial portrait, 2024Member of the House of Lords Lord TemporalIncumbentAssumed office 15 July 1991Life Peerage Personal detailsBornRobert Jacob Alexander (1939-04-25) 25 April 1939 (age 84)Harbin, Republic of ChinaNationalityBritishPolitical partyNone (Crossbench)Other politicalaffiliationsLabour (until 1981)SDP (1981–88)'Continuing' SDP (1988–90)Conservative (1992–2001)Alma materJesus College, OxfordNuffi…

Pada tahun 1776, Thomas Jefferson dalam Deklarasi Kemerdekaan Amerika Serikat mengajukan filosofi bahwa hak asasi manusia melekat pada semua orang, menegaskan bahwa semua semua orang diciptakan sederajat, bahwa mereka dikaruniai oleh Penciptanya dengan Hak-hak yang tidak dapat disangkal, dan bahwa di antara hak-hak itu adalah Kehidupan, Kemerdekaan, dan upaya mengejar Kebahagiaan. Sejarawan Joseph J. Ellis menyebut Deklarasi Kemerdekaan Amerika Serikat sebagai pernyataan hak asasi manusia dalam …

Président du gouvernement de Nouvelle-Calédonie Insigne du gouvernement de la Nouvelle-Calédonie. Titulaire actuelLouis Mapoudepuis le 16 juillet 20212 ans, 9 mois et 2 joursVice-présidente : Isabelle Champmoreau Création 28 mai 1999 Mandant Gouvernement de la Nouvelle-Calédonie Durée du mandat 5 ans[1] Premier titulaire Jean Lèques Résidence officielle Hôtel du Gouvernement de la Nouvelle-Calédonie Rémunération 838 000 XPF (7 022,44 €) brut…

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Птиц�…

Robert Guiscard de HautevilleRobert Guiscard (oleh Merry-Joseph Blondel)PasanganAlberadaSikelgaitaAnakBohemond I dari AntiokhiaEmmaRoger Borsa dari Puglia dan CalabriaRobert ScalioGuido d'Altavilla, sebastosKeluarga bangsawanWangsa HautevilleBapakTancredi d'AltavillaIbuFressendaLahirskt. 1015Cotentin, NormandiaMeninggal17 Juli 1085 (usia 70)Atheras, Lixouri utaraPemakamanSantissima Trinità, Venosa Koin Robert Guiscard. Robert Guiscard (/ɡiːˈskɑːr/;[1] Modern bahasa Prancis: [�…

Hmongic language of Guizhou, China GejiaGe, Chong'an River Miao家语, 重安江苗语Native toChinaRegionGuizhouEthnicityGejiaNative speakers(60,000 cited 1995)[1]Language familyHmong–Mien HmongicWest Hmongic? Chuanqiandian clusterGejiaDialects Gejia Dongjia Language codesISO 639-3hmjGlottologgeee1239 The Ge or Gejia language (Chinese: 家语), also known as Chong'anjiang Miao (Chinese: 重安江苗语), is a West Hmongic language of Huangping County, Guizhou, China. The …

Red-tailed hawk living in New York City (1990–2023) This article is about the hawk. For other uses, see Pall Mall. Pale MalePale Male eating a pigeon in 2011SpeciesButeo jamaicensisSexMaleHatched1990 (1990)DiedMay 16, 2023(2023-05-16) (aged 32–33)Long Island, New York, U.S.Nation fromUnited StatesKnown forNesting on 927 Fifth AvenueMate(s) First Love (1992, c. 1995–1997) Chocolate (c. 1992–1995) Blue (1998 – c. September 11, 2001) Lola (2002 – Decembe…

Hotel company based in Abu Dhabi, UAE Rotana HotelsCompany typePrivateIndustryHospitality & TourismFoundedAbu Dhabi, United Arab EmiratesFounderNasser Al Nowais & Selim El ZyrHeadquartersAbu Dhabi, United Arab EmiratesArea servedMiddle East, Africa, Balkans, TurkeyKey peoplePhilip BarnesChief Executive OfficerEddy TannousChief Operating OfficerWebsiteRotana.com Rotana Hotel Management Corporation PJSC (Arabic: روتانا) is a hotel management company in the Middle East, Africa, the Ba…

Public television network in West Virginia West Virginia Public Broadcastingstatewide West VirginiaUnited StatesChannelsDigital: See tables belowProgrammingAffiliationsPBS (1970–present)NPR (1973–present)PRIAPMBBCAPTOwnershipOwnerWest Virginia Educational Broadcasting AuthorityHistoryFirst air dateJuly 14, 1969; 54 years ago (1969-07-14)Former affiliationsNET (1969–1970)Call sign meaningSee tables belowTechnical informationFacility IDSee tables belowERPSee tables belowHAA…

Asian Highway 42 (AH42) adalah bagian dari Jaringan Jalan Asia, sejauh 3.754 kilometer (2.333 mi) dari AH5 di Lanzhou, Tiongkok[1] ke AH1 di Barhi, India.[2] Jalur ini melewati China, Nepal,[3] dan India. yang melewati Gunung Everest. Setengah jalur dari ini yang membentang dari Lhasa ke Lanzhou di Tiongkok dikenal sebagai Rencana Asian Highway.[4] China G109: Lanzhou - Xining - Golmud - Lhasa G318: Lhasa - Zhangmu Nepal Tribhuvan Highway: Kathmandu - Narayan…

Pearl JamPearl Jam pada tahun 2006, kiri ke kanan: Mike McCready, Jeff Ament, Matt Cameron, Eddie Vedder dan Stone GossardInformasi latar belakangNama lainMookie BlaylockAsalSeattle, Washington, Amerika SerikatGenreAlternative rock, grunge, hard rockTahun aktif1990–sekarangLabelEpic, JArtis terkaitGreen River, Soundgarden, Bad Radio, Mother Love Bone, Temple of the Dog, Brad, Wellwater Conspiracy, Mad Season, Three Fish, The RockfordsSitus webwww.pearljam.comAnggotaJeff AmentStone GossardMike …

Marvel Comics superhero Comics character FlatmanFlatman as depicted in G.L.A. #2 (July 2005). Art by Paul Pelletier.Publication informationPublisherMarvel ComicsFirst appearanceThe West Coast Avengersvol. 2 #46 (July 1989)Created byJohn ByrneIn-story informationAlter egoMatt (surname unrevealed)[1]SpeciesHuman mutantTeam affiliationsGreat Lakes AvengersNotable aliasesThe 2-D DefenderDr. Val VenturaAbilities Origami shapeshifting Flat body Elasticity Flatman (Matt) is a superhero appearin…

6th Central Committee← 5th7th →Emblem of the League of Communists of Yugoslavia7 November 1952 – 26 April 1958(5 years, 170 days)OverviewTypeHighest organElection6th CongressMembersTotal109 membersNewcomers50 members (7th)Old58 members (5th)Reelected103 members (7th) This electoral term of the Central Committee was elected by the 6th Congress of the League of Communists of Yugoslavia in 1952, and was in session until the convocation of the 7th Congress in 1958. …

此條目可能包含不适用或被曲解的引用资料,部分内容的准确性无法被证實。 (2023年1月5日)请协助校核其中的错误以改善这篇条目。详情请参见条目的讨论页。 各国相关 主題列表 索引 国内生产总值 石油储量 国防预算 武装部队(军事) 官方语言 人口統計 人口密度 生育率 出生率 死亡率 自杀率 谋杀率 失业率 储蓄率 识字率 出口额 进口额 煤产量 发电量 监禁率 死刑 国债 外…

Cette carte de 1856 montre les États esclavagistes (gris), les États abolitionnistes (rouge) et les territoires américains (vert) avec le Kansas (non coloré). La loi Kansas-Nebraska (Kansas-Nebraska Act) du 30 mai 1854 crée les territoires du Kansas et du Nebraska, organise des terres nouvelles, abroge le Compromis du Missouri de 1820, et permet aux immigrants installés dans ces territoires de décider si oui ou non ils y introduiront l’esclavage. À l’origine, l’objectif de cet Acte…


















