|
CD28 family receptor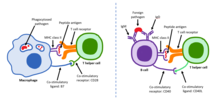 CD28 family receptors are a group of regulatory cell surface receptors expressed on immune cells. The CD28 family in turn is a subgroup of the immunoglobulin superfamily.[1] Two family members, CD28 and ICOS, act as positive regulators of T cell function while another three, BTLA,[2] CTLA-4 and PD-1 act as inhibitors.[1] Ligands for the CD28 receptor family include B7 family proteins.[3] CD28 receptors play a role in the development and proliferation of T cells.[4] The CD28 receptors enhance signals from the T cell receptors (TCR) in order to stimulate an immune response[5] and an anti-inflammatory response on regulatory T cells. Through the promotion of T cell function, CD28 receptors allow effector T cells to combat regulatory T cell-mediated suppression from adaptive immunity. CD28 receptors also elicit the prevention of spontaneous autoimmunity. FunctionCD28 receptors aid in other T cell processes such as cytoskeletal remodeling, production of cytokines and chemokines and intracellular biochemical reactions (i.e. phosphorylation, transcriptional signaling, and metabolism) that are key for T cell proliferation and differentiation. Ligation of CD28 receptors causes epigenetic, transcriptional and post-translational alterations in T cells. Specifically, CD28 costimulation controls many aspects within T cells, one being the expression of proinflammatory cytokine genes. A particular cytokine gene encodes for IL-2, which influences T cell proliferation, survival, and differentiation. The absence of CD28 costimulation results in the loss of IL-2 production causing the T cells to be anergic.[6] Additionally, CD28 ligation causes arginine-methylation for many proteins. CD28 also drives transcription within T cells and produce signals that lead to IL-2 production and Bcl-xL regulation, an antiapoptotic protein, which are essential for T cell survival.[7] CD28 receptors can be seen on 80% of human CD4+ and 50% of CD8+ T cells, in which this percentage decreases with age.[8] Clinical significanceCancerSome cancer cells evade destruction by the immune system through an overexpression of B7 ligands that bind to inhibitory CD28 family member receptors on immune cells.[9] Antibodies directed against CD28 family members CTLA-4, PD-1, or their B7 ligands function as checkpoint inhibitors to overcome tumor immune tolerance and are clinically used in cancer immunotherapy.[9] Additionally, genetically engineered T cells containing CD28 and CD137 can be used in a molecularly targeted therapy response to a type of carcinomas called mesothelin. These T cells have a high affinity for human mesothelin. Upon mesothelin stimulation, the T cells proliferate, express an antiapoptotic gene, and secrete cytokines with the help of CD28 expression. When introduced to mice with pre-existing tumors, these T cells remove the tumors completely. The CD137 presence within the cells maintains the persistence of the engineered T cells. This interaction between engineered T cells with CD28 and CD137 are essential for immunotherapy, and show promise for directing T lymphocytes to tumor antigens and altering the tumor microenvironment for mesothelin.[10] HIVThe CD28 pathway is targeted by the human immunodeficiency virus (HIV) as the virus infects large numbers of normal cells. CD28 has effects on the transcription and stability of interleukin-2 and IFN-γ, cytokines that are important for immunity and stimulating NK cells. HIV alters the CD28 signaling as well as CD8 cells. As a result, there are reduced levels of CD8 cells, which express CD28, in individuals with HIV. With regards to subjects with both Hepatitis C Virus (HCV) and HIV, levels of CD8 cells are also reduced. CD28 signaling has a large role in the adaptive response to HCV and can increase morbidity for HCV/HIV coinfection within a subject.[11] CD28 induces IL-2 secretion that increases IL-2 mRNA stability. CD28 costimulation influences the expression of key genes expressed in T cell differentiation. Tat, a regulatory protein that regulates viral transcription, increases the transcription of the HIV dsDNA. CD28 costimulation with the Tat protein can contribute to chronic immune hyperactivation seen among HIV-infected individuals. Thus, CD28 is an essential part of therapeutics for the infection and pathogenesis of HIV.[12] Hyper-induced inflammatory cytokinesBinding CD28 to superantigens can induce an overexpression of inflammatory cytokines which may be harmful. When CD28 interacts with coligand B7-2, these superantigens elicit T-cell hyperactivation. Superantigens can form this overexpression by controlling interactions between MHC-II and TCRs as well as increasing the B7-2 and CD28 costimulatory interactions. This is dangerous because the overexpression of inflammatory cytokines can cause toxic shock in an individual.[13] References
|