Food and Drug Administration Safety and Innovation Act
| |||||||||||||||||||||
Read other articles:

Milan Babić (26 Februari 1956 – 5 Maret 2006) adalah seorang pemimpin perang etnis Serbia dari tahun 1991 hingga 1995. Ia merupakan pemimpin Republik Krajina Serbia, sebuah kawasan mayoritas etnis Serbia yang memecahkan diri dari Kroasia pada 1991. Pada 2004, Pengadilan Internasional bagi Penjahat Perang memvonis Babić dengan hukuman 13 tahun penjara. Ia ditemukan tewas bunuh diri di sel tahanannya di Den Haag pada 5 Maret 2006. Milan Babić Presiden Republik Krajina Serbia ke-…

Ino いの町Kota kecil BenderaLambangLokasi Ino di Prefektur KōchiNegara JepangWilayahShikokuPrefektur KōchiDistrikAgawaPemerintahan • Wali kotaMakiko IkedaLuas • Total471 km2 (182 sq mi)Populasi (Oktober 1, 2015) • Total22.767 • Kepadatan48,34/km2 (12,520/sq mi)Zona waktuUTC+09:00 (JST)Kode pos781-2192Simbol • PohonEdgeworthia chrysantha • BungaRhododendron • BurungSit…

Initiation LovePosterNama lainイニシエーション・ラブSutradaraYukihiko TsutsumiPemeranAtsuko MaedaShota MatsudaFumino KimuraPerusahaanproduksiOffice CrescendoDistributorTohoTanggal rilis 23 Mei 2015 (2015-05-23) Durasi110 menitNegaraJepangBahasaJepangPendapatankotor¥1,31 miliar (Jepang) Initiation Love (イニシエーション・ラブcode: ja is deprecated ) adalah film percintaan Jepang 2015 yang disutradarai oleh Yukihiko Tsutsumi. Film tersebut dirilis di Jepang pada 2…

Dany Budiyanto Inspektur Pusat Teritorial Angkatan DaratPetahanaMulai menjabat 31 Mei 2023 PendahuluJoseph Robert GiriPenggantiPetahanaKepala Staf Komando Daerah Militer XIV/HasanuddinMasa jabatan21 Januari 2022 – 31 Mei 2023 PendahuluAmping Bujasar TangdilintinPenggantiMohammad Syech Ismed Informasi pribadiLahir3 Januari 1971 (umur 53)IndonesiaAlma materAkademi Militer (1992)Karier militerPihak IndonesiaDinas/cabang TNI Angkatan DaratMasa dinas1992—sekarangPangka…

Species of seaweed Caulerpa brownii Scientific classification (unranked): Viridiplantae Division: Chlorophyta Class: Ulvophyceae Order: Bryopsidales Family: Caulerpaceae Genus: Caulerpa Species: C. brownii Binomial name Caulerpa brownii(C.Agardh) Endl. Caulerpa brownii is a species of seaweed in the Caulerpaceae family. Distribution This species can be found along the southern North Island, the South Island, Chatham Island, Stewart Island, Snares Island of New Zealand.[1] It is also…

Digital payments network For other uses, see Zelle (disambiguation). ZelleFormerlyclearXchangeCompany typePrivateIndustryPayment servicePayment networkMobile appFounded2016; 8 years ago (2016)HeadquartersScottsdale, Arizona, United StatesServicesElectronic funds transferParentEarly Warning Services, LLCWebsitewww.zellepay.com Zelle (/zɛl/) is a United States–based digital payments network run by a private financial services company owned by the banks Bank of America, Truist,…

Untuk kegunaan lain, lihat Filipus. PhilipPatung Rasul Filipus di Katedral Saint Isaac Saint Petersburg, RussiaRasul dan MartirLahirTidak diketahuiBethsaida, GalileeMeninggalc.80Hierapolis, dengan disalibkanKanonisasiPre-congregationPesta3 Mei (Gereja Katolik Roma), 14 November (Eastern Orthodox Church), May 1 (Anglican Communion, Lutheran Church and pre-1955 General Roman Calendar), 11 May (General Roman Calendar, 1955–69)AtributElderly bearded, Saint, and open to God man holding a basket of …

Samjin Company English ClassPoster rilis teatrikalNama lainHangul삼진그룹 영어토익반 Alih Aksara yang DisempurnakanSamjingeurup Yeongeotoikban SutradaraLee Jong-pilProduserPark Eun-kyungSkenarioLee Jong-pilPemeranGo Ah-sungEsomPark Hye-suPenata musikDalpalanSinematograferPark Se-seungPenyuntingHeo Sun-miJo Han-ulPerusahaanproduksiThe LAMPDistributorLotte EntertainmentTanggal rilis 21 Oktober 2020 (2020-10-21) Durasi110 menitNegaraKorea SelatanBahasaKoreaPendapatankotorp…

Cheltenham TownNama lengkapCheltenham Town Football ClubJulukanThe RobinsBerdiri1887StadionWhaddon Road Cheltenham(Kapasitas: 7,066)KetuaDavid BloxhamManajerDarrell ClarkeLigaLiga Satu Inggris2022–23ke-16, Liga Satu Inggris Kostum kandang Kostum tandang Musim ini Cheltenham Town Football Club adalah sebuah klub sepak bola Inggris yang berbasis di kota Cheltenham, Gloucestershire dan saat ini bermain di Liga Satu Inggris, tingkat ke-3 dalam sistem liga sepak bola Inggris. Didirikan pada tahun 1…

Belarusian artist (1866–1924) Léon BakstBakst's Self-portrait, 1893, oil on cardboard, 34 × 21 cm, The State Russian Museum, St. Petersburg, RussiaBornLeyb-Khaim Izrailevich Rosenberg27 January 1866Grodno, Grodno Governorate, Russian EmpireDied27 December 1924 (aged 58)[1]Rueil-Malmaison, near ParisNationalityRussianEducationSt. Petersburg Academy of ArtsMovementModernist, Orientalist themes Léon Bakst, born Leyb-Khaim Izrailevich Rosenberg (Russian: Леон (Лев) Самойло…
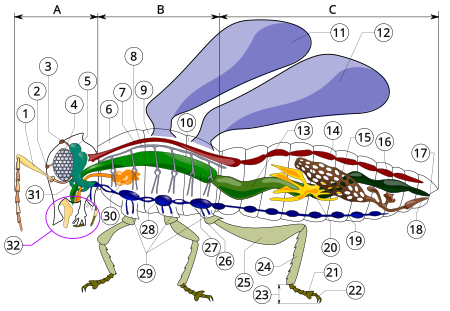
Serangga Periode Awal Devon[1] – sekarang 396–0 jtyl PreЄ Є O S D C P T J K Pg N Insecta Searah jarum jam dari kiri atas: Empis livida, Rhinotia hemistictus, anjing tanah (Gryllotalpa brachyptera), Vespula germanica, Opodiphthera eucalypti, HarpactorinaeTaksonomiSuperkerajaanEukaryotaKerajaanAnimaliaFilumArthropodaKelasInsecta Linnaeus, 1758 SubkelasArchaeognatha †Coxoplectoptera Dicondylialbs Serangga merupakan hewan yang membentuk kelas Insekta (berasal dari bahasa Latin: …

Chronologie de la France ◄◄ 1763 1764 1765 1766 1767 1768 1769 1770 1771 ►► Chronologies Procès-verbal d’examen du corps de la bête du Gévaudan adressé à l’intendant d’Auvergne le 20 juin 1767, première page.Données clés 1764 1765 1766 1767 1768 1769 1770Décennies :1730 1740 1750 1760 1770 1780 1790Siècles :XVIe XVIIe XVIIIe XIXe XXeMillénaires :-Ier Ier IIe IIIe Chronologies thématiques Art Architecture, Ar…

المعاهد العليا بكينج مريوط شعار المعاهد العليا بكينج مريوط معلومات المؤسس الدكتور/ يوسف درويش خليل التأسيس 1996 (منذ 28 سنة) النوع مؤسسة تعليمية خاصة المعاهد المعهد العالي للهندسة والتكنولوجيا المعهد العالي للسياحة والفنادق المعهد العالي للدراسات الأدبية المعهد العالي للحاس…

This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: List of mergers in Kagoshima Prefecture – news · newspapers · books · scholar · JSTOR (July 2013) (Learn how and when to remove this template message) Here is a list of mergers in Kagoshima Prefecture, Japan since the Heisei period. Mergers from April 1, 1999 to present On October …

Berhias california Nama lain Spangle Asal California, Amerika Serikat Standar ras TICA standar Kucing domestik (Felis catus) Kucing berhias california (Inggris: California Spangled cat) adalah salah satu ras kucing yang langka dan mahal yang juga memiliki bulu berpola macan tutul. Kucing berhias california merupakan hasil persilangan dari ras kucing domestik dengan bulu berpola macan tutul.[1] Sejarah Ras berhias california diciptakan oleh seorang antropologi dari California bernama …

Vektor basis beralih ke halaman ini. Untuk vektor basis dalam konteks kristal, lihat Struktur kristal. Untuk konsep yang lebih umum dalam fisika, lihat Kerangka acuan. Vektor yang sama (panah berwarna biru tua) dapat dinyatakan dengan menggunakan dua basis yang berbeda (panah-panah berwarna ungu dan berwarna merah). Dalam matematika, sebarang himpunan vektor B dalam suatu ruang vektor V disebut basis, jika setiap elemen di V dapat dituliskan sebagai kombinasi linear terhingga yang unik dari elem…

Questa voce sull'argomento cestisti statunitensi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Cumberland Posey Posey Cumberland nel 1913 Nazionalità Stati Uniti d'America Altezza 175 cm Peso 66 kg Pallacanestro Ruolo Guardia Termine carriera 1925 Hall of fame Naismith Hall of Fame (2016) Carriera Squadre di club Monticello Athletic AssociationMonticello-Delany RiflesLoendi Big FiveMonarch El…

Ninja Cilik HattoriHattori-kun忍者ハットリくん(Ninja Hattori-kun)GenreAksi, petualangan, komedi, seni bela diri AnimeSutradaraBerbagai sutradaraStudio Shin-Ei Animation IMMG(7 Juli 1997-23 Februari 2003)Tayang Ninja Hattori-kun:28 September 1981 – 25 Desember 1987Ninja Hattori-kun Returns:13 Mei 2013 – sekarang Portal anime dan manga Ninja Cilik Hattori (忍者ハットリくんcode: ja is deprecated , Ninja Hattori-kun) adalah serial manga karya Fujiko Fujio A (nama asli: …

French royal château Palace of FontainebleauChâteau de FontainebleauInteractive fullscreen mapLocationFontainebleau, Seine-et-Marne, FranceCoordinates48°24′8″N 2°42′2″E / 48.40222°N 2.70056°E / 48.40222; 2.70056 UNESCO World Heritage SiteOfficial namePalace and Park of FontainebleauTypeCulturalCriteriaii, viDesignated1981 (5th session)Reference no.160UNESCO RegionEurope and North America Palace of Fontainebleau (/ˈfɒntɪnbloʊ/ FON-tin-bloh, US also /…

Cette page concerne l'année 1730 du calendrier grégorien. Pour l'année 1730 av. J.-C., voir 1730 av. J.-C. Chronologies Méntouab (Mentewab), régente d'Éthiopie, sur une fresque de Narga Selassie, aux pieds de Marie et de l'enfant Jésus. 1748.Données clés 1727 1728 1729 1730 1731 1732 1733Décennies :1700 1710 1720 1730 1740 1750 1760Siècles :XVIe XVIIe XVIIIe XIXe XXeMillénaires :-Ier Ier IIe IIIe Chronologies thématiques…
