Supercritical carbon dioxide
|

Daiki Ogawa Informasi pribadiNama lengkap Daiki OgawaTanggal lahir 16 Oktober 1991 (umur 32)Tempat lahir Prefektur Shizuoka, JepangPosisi bermain BekKarier senior*Tahun Tim Tampil (Gol)2014- Júbilo Iwata * Penampilan dan gol di klub senior hanya dihitung dari liga domestik Daiki Ogawa (lahir 16 Oktober 1991) adalah pemain sepak bola asal Jepang. Karier Daiki Ogawa pernah bermain untuk Júbilo Iwata. Pranala luar (Jepang) Profil dan statistik di situs web resmi J. League Data Site Artikel b…

MINI La Mini nuova in confronto con l'antenata Descrizione generale Costruttore Mini Tipo principale berlina 2 volumi Produzione dal 2001 Sostituisce la Mini (1959) Altre caratteristiche Altre antenate Rover serie 100 La Mini è un'automobile, rivisitazione in chiave moderna della Mini del 1959, prodotta dal 2001 sotto l'egida del costruttore tedesco BMW, e atta a ricoprire il segmento B. Indice 1 Genesi 1.1 Le concept E1 e ACV30 1.2 Il modello definitivo 2 Prima serie (R50, R52, R53, 2001…

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Marcelo Peralta – berita · surat kabar · buku · cendekiawan · JSTOR (March 2020) Marcelo Peralta (5 Maret 1961 – 10 Maret 2020)) adalah pemain musik, guru, komposer, dan arranger dari Argentina y…

The Japanese Wifeザ ・ジャッパニーズ ・ワイフPosterSutradaraAparna SenSkenarioAparna SenCeritaKunal BasuPemeranRahul BoseRaima SenMoushumi ChatterjeeChigusa TakakuPenata musikSagar DesaiSinematograferAnay GoswamyPenyuntingRaviranjan MaitraDistributorSaregama FilmsTanggal rilis 9 April 2010 (2010-04-09) [1]Durasi105 menitNegaraIndia, JepangBahasaInggris, Bengali, Jepang The Japanese Wife (Jepang: ザ ・ジャッパニーズ ・ワイフ) adalah sebuah film India p…

American television writer and producer For the keyboardist, see Bradley Bell (musician). This article uses bare URLs, which are uninformative and vulnerable to link rot. Please consider converting them to full citations to ensure the article remains verifiable and maintains a consistent citation style. Several templates and tools are available to assist in formatting, such as reFill (documentation) and Citation bot (documentation). (August 2022) (Learn how and when to remove this template messa…

Halaman ini berisi artikel tentang rumpun bahasa Finnik Baltik. Untuk cabang yang lebih tinggi, lihat Rumpun bahasa Finno-Permik. Cari artikel bahasa Cari berdasarkan kode ISO 639 (Uji coba) Cari berdasarkan nilai Glottolog Kolom pencarian ini hanya didukung oleh beberapa antarmuka Halaman rumpun acak Rumpun bahasaFinnik Finnik Baltik, Balto-FinnikEtnisSuku-suku Balto-FinnikPersebaranFennoskandia Utara, Estonia, Rusia Barat Laut, Latvia, Rusia Barat Daya dan TenggaraPenggolon…

Lambang negara Andorra (Catalan: Escut d'Andorra) Berupa perisai yang terbagi menjadi empat bagian yang terdiri atas lambang dari Bishop of Urgell, Lambang Count of Foix, Lambang Catalonia serta lambang Viscounts of Béarn. Dimanfaatkan secara tidak resmi sejak Abad Pertengahan, statusnya sebagai lambang negara Kerajaan Andorra diformalkan pada tahun 1993 setelah penerapan konstitusi baru mereka. Lambang ini juga muncul pada bendera Andorra. Pranala luar Official website of the Government of Alg…

This is a list of accidents and incidents involving the Douglas DC-3A that have taken place in the period 1980–1989, including aircraft based on the DC-3 airframe such as the Douglas C-47 Skytrain and Lisunov Li-2. Military accidents are included; and hijackings and incidents of terrorism are covered, although acts of war are outside the scope of this list. 1980 5 January In Canada, Douglas DC-3 C-FBKX of Lambair was damaged at Thompson Airport in Thompson, Manitoba. The landing gear retra…

Kampfgruppen der ArbeiterklasseBendera KampfgruppenAktif29 September 1953 – 14 December 1989Negara Republik Demokratik JermanAliansiPakta WarsawaJumlah personel202,000–211,000, masa damai 1980[1]Bagian dariKementerian Dalam Negeri Kelompok Penyerang Kelas Buruh (bahasa Jerman: Kampfgruppen der Arbeiterklasse, KdA) adalah sebuah organisasi paramiliter di Jerman Timur. Organisasi tersebut didirikan pada tahun 1953 dan dibubarkan pada tahun 1990. Referensi ^ Torsten Diedrich, Hans Ehler…

Dari Kiri Atas: Cakrawala pusat kota seperti yang terlihat dari jembatan di atas Sungai Flint di kampus Universitas Michigan- Flint, GM Powertain dan Lapangan Golf Swartz Creek, Planetarium Longway, Bekas Situs Buick City, South Saginaw Street, dan Bank Warga Bola Cuaca. Flint ialah sebuah kota industri di Michigan, Amerika Serikat. Berpenduduk 124.943 jiwa (2000). Kota ini telah mendapatkan perhatian tertentu di luar Amerika Serikat melalui produksi Michael Moore. Kota ini banyak berubah akibat…

L'Arc en CielKonser L'Arc~en~Ciel di Madison Square Garden, 2012. Dari kiri ke kanan: Tetsuya, Hyde, Yukihiro dan Ken.Informasi latar belakangNama lainLarukuAsal Osaka, JepangGenreMusik pop, Alternative rock, Hard rock, Progressive rockTahun aktif1991-sekarangLabelKi-Oon (Jepang), Sony, Tofu, Danger CrueSitus webwww.larc-en-ciel.comAnggotaHydeTetsuyaKenYukihiroMantan anggotaSakuraHiroPero L'Arc~en~Ciel (ラルク アン シエルcode: ja is deprecated , Raruku An Shieru, Pelangi dalam bahasa Pra…

Replika Bounty, yang dibangun pada 1960 HMS Bounty, juga disebut sebagai HM Kapal Bersenjata Bounty, adalah sebuah kapal niaga kecil yang dibeli oleh Angkatan Laut Britania Raya untuk misi botani. Kapal tersebut dikirim ke Samudera Pasifik di bawah komando William Bligh untuk mengangkut tumbuhan-tumbuhan sukun dan membawanya ke wilayah-wilayah Britania di Hindia Barat. Misi tersebut tak pernah rampung karena dahagi yang dipimpin oleh Pelaksana Jabatan Letnan Fletcher Christian. Insiden tersebut …
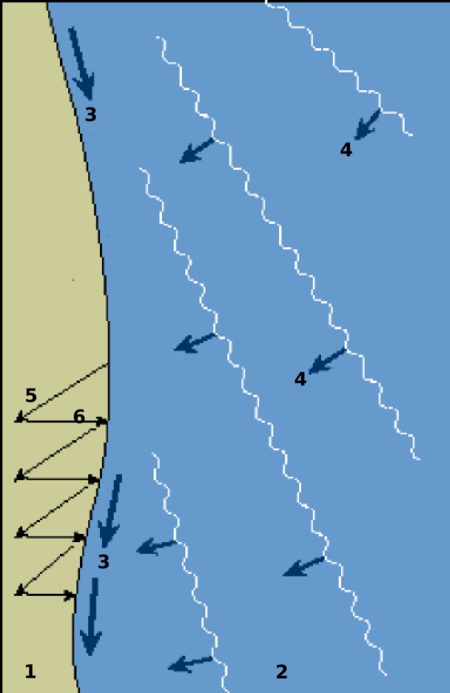
Diagram proses-proses pantai yang berkaitan dengan arus sejajar pantai1=pantai2=laut3=arah arus sejajar pantai4=gelombang datang5=arah sedimen yang terbawa naik oleh gelombang6=arah sedimen ketika terbawa oleh air yang surut Arus sejajar pantai atau arus susur pantai (bahasa Inggris: longshore current) adalah serangkaian proses geologis yang menyebabkan terjadinya perpindahan sedimen (seperti tanah liat, lanau, kerikil, atau pasir) di sepanjang garis pantai. Gelombang atau angin yang datang deng…

Cover di Majalah Time Alfred Pritchard Sloan, Jr. (23 Mei 1875 – 17 Februari 1966) merupakan seorang pendiri dan pemimpin General Motors pada awal abad ke-20. Dia dilahirkan di New Haven, Connecticut. Dia melanjutkan pendidikannya di Institut Teknologi Massachusetts pada tahun 1892. Dia memimpin General Motors dari tahun 1923 hingga 1956. Meninggal dunia pada 17 Februari 1966. Pranala luar Alfred P. Sloan Foundation, whose total assets had a market value of over $1.5 billion in 2…

Ama-no-Uzume (Jepang: 天宇受売命 atau 天鈿女命) adalah dewi tari dan kebahagiaan, atau dewi yang menari pada pagi hari.[1] Dengan bermandikan air yang mengucur di atas guci, Ama-no-Uzume menari-nari demi menghibur para dewa-dewi di surga.[2] Tarian Ama-no-Uzume sering dilakukan dalam pentas Kagura pada era modern.[3] Tablet Ama-no-Uzume di kuil Takachiho. Sekilas Ama no Uzume artinya adalah “dewi aroma surga” dan terkadang dijuluki juga Ame-no-Uzume-no-Mi…

Jean ParkerParker pada 1934LahirLois May Green(1915-08-11)11 Agustus 1915Deer Lodge, Montana, A.S.Meninggal30 November 2005(2005-11-30) (umur 90)Los Angeles, California, A.S.MakamForest Lawn Memorial Park (Hollywood Hills)Tahun aktif1932–1966Suami/istriGeorge MacDonald (m. 1936; c. 1940) Douglas Dawson (m. 1941; c. 1943) Curtis Grotter (m. 1944; …

Adi UtariniAdi Utarini, Anggota Dewan Pengarah BRIN (2021)AlmamaterUniversitas Umeå UCL Great Ormond Street Institute of Child Health Universitas Gadjah MadaDikenal atasUji terkontrol secara acak terhadap teknologi Wolbachia dalam pemberantasan demam berdarah denguePenghargaanNature's 10 (2020), Time 100 (2021)Karier ilmiahInstitusiUniversitas Gadjah MadaDisertasiEvaluation of the user-provider interface in malaria control programme : the case of Jepara district, Central Java province, Ind…

The Battles and Operations involving the Indian National Army during World War II were all fought in the South-East Asian theatre. These range from the earliest deployments of the INA's preceding units in espionage during Malayan Campaign in 1942, through the more substantial commitments during the Japanese Ha Go and U Go offensives in the Upper Burma and Manipur region, to the defensive battles during the Allied Burma campaign. The INA's brother unit in Europe, the Indische Legion did not see a…

A. J. Foyt driving a Championship Car in 1984 From 1956 to 1978, the United States Auto Club (USAC) sanctioned Championship Car class featured the top teams and drivers in U.S. open-wheel racing. Until 1971, raves included road courses, ovals, dirt courses, and, on occasion, a hill climb. Thereafter, the schedule consisted mainly of paved ovals. In 1979, the majority of car owners left the USAC to race under the auspices of Championship Auto Racing Teams (CART). This led to a decline in the numb…

Artikel atau sebagian dari artikel ini mungkin diterjemahkan dari List of Padma Vibhushan award recipients di en.wikipedia.org. Isinya masih belum akurat, karena bagian yang diterjemahkan masih perlu diperhalus dan disempurnakan. Jika Anda menguasai bahasa aslinya, harap pertimbangkan untuk menelusuri referensinya dan menyempurnakan terjemahan ini. Anda juga dapat ikut bergotong royong pada ProyekWiki Perbaikan Terjemahan. (Pesan ini dapat dihapus jika terjemahan dirasa sudah cukup tepat. Lihat …
