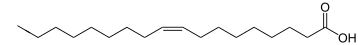
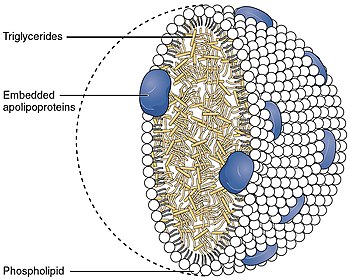
Triglyceride
|
Read other articles:
Hitzhusen Lambang kebesaranLetak Hitzhusen di Segeberg NegaraJermanNegara bagianSchleswig-HolsteinKreisSegeberg Municipal assoc.Bad Bramstedt-LandPemerintahan • MayorHorst-Günther HungerLuas • Total7,93 km2 (306 sq mi)Ketinggian24 m (79 ft)Populasi (2013-12-31)[1] • Total1.258 • Kepadatan1,6/km2 (4,1/sq mi)Zona waktuWET/WMPET (UTC+1/+2)Kode pos24576Kode area telepon04192Pelat kendaraanSESitus webwww.amt-b…

Charles Hippolyte Louis Jules BinetLahir(1868-07-04)4 Juli 1868ClamecyMeninggal14 November 1941(1941-11-14) (umur 73)NiceKebangsaanPrancisPekerjaanDokter, PsikologKarya terkenalLa Folie de JésusLe Haras humain Charles Binet-Sanglé (4 Juli 1868 – 14 November 1941) adalah seorang dokter militer dan psikolog asal Prancis. Ia dikenal sebagai orang pertama yang secara terbuka mempertanyakan kesehatan mental Yesus, yang ia cantumkan dalam buku La Folie de Jésus.[1][…

artikel ini perlu dirapikan agar memenuhi standar Wikipedia. Tidak ada alasan yang diberikan. Silakan kembangkan artikel ini semampu Anda. Merapikan artikel dapat dilakukan dengan wikifikasi atau membagi artikel ke paragraf-paragraf. Jika sudah dirapikan, silakan hapus templat ini. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) CibarusahKecamatanNegara IndonesiaProvinsiJawa BaratKabupatenBekasiPemerintahan • CamatMuhamad KurnaepiPopulasi • Total7…

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Universitas UCSI, Kampus Sarawak – berita · surat kabar · buku · cendekiawan · JSTOR Artikel atau sebagian dari artikel ini mungkin diterjemahkan dari UCSI University, Sarawak Campus di en.wikipedia.org. Isinya …

Charles Evans Whittaker Hakim Mahkamah Agung Amerika SerikatMasa jabatan25 Maret 1957 – 31 Maret 1962 Informasi pribadiKebangsaanAmerika SerikatProfesiHakimSunting kotak info • L • B Charles Evans Whittaker adalah hakim Mahkamah Agung Amerika Serikat. Ia mulai menjabat sebagai hakim pada mahkamah tersebut pada tanggal 25 Maret 1957. Masa baktinya sebagai hakim berakhir pada tanggal 31 Maret 1962.[1] Referensi ^ Justices 1789 to Present. Washington, D.C.: Mahkamah…

Untuk kegunaan lain, lihat Bandar Udara Internasional New York. Bandar Udara Internasional John F. KennedyIATA: JFKICAO: KJFKFAA LID: JFKWMO: 74486InformasiJenisPublikPemilikKota New York[1]PengelolaPort Authority of New York and New Jersey[1]MelayaniKota New YorkLokasiJamaica, Queens, Kota New York, Amerika SerikatMaskapai penghubung Penumpang American Airlines Delta Air Lines JetBlue Airways Kargo Evergreen International Airlines Polar Air Cargo Kalitta Air Ketinggian dpl4…

2012 2022 Élections législatives de 2017 dans la Vendée 5 sièges de députés à l’Assemblée nationale 11 et 18 juin 2017 Campagne 22 mai au 10 juin12 juin au 16 juin Corps électoral et résultats Population 662 122 Inscrits 514 528 Votants au 1er tour 266 979 53,18 % 7,2 Votes exprimés au 1er tour 266 979 Votes blancs au 1er tour 4 475 Votes nuls au 1er tour 2 176 Votants au 2d tour 227 382 44,19 % Votes exprimés au …

Orang SamoaJumlah populasic. 650,000-700,000Daerah dengan populasi signifikan Samoa194,320 Amerika Serikat184,440[1] Selandia Baru144,138[2] Australia75,755[3] Samoa Amerika55,519 Kanada1,100[4]BahasaSamoa, InggrisAgamaKekristenan, Politeisme Orang Samoa (bahasa Samoa: tagata Sāmoa) adalah penduduk asli Polinesia di Kepulauan Samoa, sebuah kepulauan di Polinesia. Mereka menuturkan bahasa Samoa. Secara politis dan geografis, kepul…

1929 film The Great DivideDirected byReginald BarkerWritten byScenario, dialogue, intertitles:Fred MytonPaul PerezBased onThe Great Divide1906 playby William Vaughn MoodyProduced byRobert North (uncredited)CinematographyLee GarmesAlvin KnechtelProductioncompanyFirst National PicturesDistributed byWarner Bros.Release dates September 15, 1929 (1929-09-15) (Vitaphone sound) October 27, 1929 (1929-10-27) (silent) Running time72 minutesCountryUnited StatesLanguag…

هارتويك الإحداثيات 42°39′35″N 75°02′56″W / 42.659722222222°N 75.048888888889°W / 42.659722222222; -75.048888888889 [1] تقسيم إداري البلد الولايات المتحدة[2] التقسيم الأعلى مقاطعة أوتسيغو خصائص جغرافية المساحة 40.42 ميل مربع ارتفاع 508 متر عدد السكان عدد السكان 1952 …

MalukKecamatanNegara IndonesiaProvinsiNusa Tenggara BaratKabupatenSumbawa BaratPemerintahan • Camat-Populasi • Total11.952 jiwaKode Kemendagri52.07.08 Kode BPS5207021 Luas- km²Desa/kelurahan5 desa Maluk adalah sebuah kecamatan di Kabupaten Sumbawa Barat, Nusa Tenggara Barat, Indonesia. Daerah maluk adalah salah satu dari desa lingkar tambang yang di kelola PT. Amman mineral. Daerah maluk adalah tempatnya keregaman suku berpenghuni, karena kebanyakan penduduknya bera…

Partai Emir Faisal di Versailles, selama Konferensi Perdamaian Paris 1919. Di tengah, dari kiri ke kanan: Rustum Haidar, Nuri as-Said, Pangeran Feisal, Kapten Pisani (di belakang Feisal), T.E. Lawrence, budak Feisal (nama tidak diketahui), Kapten Tahsin Qadri Konferensi Perdamaian Paris tahun 1919 adalah konferensi yang dilaksanakan oleh pemenang Perang Dunia I untuk menegosiasikan perjanjian perdamaian antara Sekutu dan Blok Sentral yang telah kalah. Konferensi ini dibuka di Istana Versailles p…

A Motorola T-1300 series remote control is built in a telephone housing. The telephone dial is replaced with a metal plate on which is mounted a speaker, volume control and option switches. This remote control uses a two-wire circuit to control a base station. A General Electric MASTR II remote control and desk microphone. Remote controls are used any time a two-way radio base station is located away from the desk or office where communication originates. For example, a dispatch center for taxic…

Indian cricketer (born 1993) In this Indian name, the name Ramaswamy Gopal is a patronymic, and the person should be referred to by the given name, Shreyas. Shreyas GopalGopal in a post-match presentation during 2019 IPLPersonal informationFull nameRamswamy Shreyas GopalBorn (1993-09-04) 4 September 1993 (age 30)Bangalore, Karnataka, IndiaBattingRight-handedBowlingRight-arm leg breakRoleAll-rounderDomestic team information YearsTeam2013/14–2022/23Karnataka2014–2017Mumbai Indians (s…

Hungarian politician (1904–1965) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Mihály Farkas – news · newspapers · books · scholar · JSTOR (January 2017) (Learn how and when to remove this message) The native form of this personal name is Farkas Mihály. This article uses Western name order when mentio…

Operating system for the Nintendo Switch video game consoleThis article's lead section may be too short to adequately summarize the key points. Please consider expanding the lead to provide an accessible overview of all important aspects of the article. (August 2023)Some of this article's listed sources may not be reliable. Please help improve this article by looking for better, more reliable sources. Unreliable citations may be challenged and removed. (March 2024) (Learn how and when to remove …

ExyteCompany typeGmbHIndustryBuilding Design, Engineering, Procurement and ConstructionPredecessorM+W GroupFounded1912HeadquartersStuttgart, GermanyKey people Wolfgang Büchele (CEO) Peter Schönhofer (CFO) Georg Stumpf (Chairman of the supervisory board) Revenue€4.9 billion[1][2] (2021)Number of employees7400[1] (end of 2021)Websitewww.exyte.net Exyte is an international company for design, engineering, procurement, and construction in controlled and reg…

Town in Greater Amman area with Cave of the Seven Sleepers For the town in Ajloun Governorate, see Rajeb. Place in Amman Governorate, JordanAl-Rajib الرجيبAl-RajibLocation in JordanCoordinates: 31°54′N 35°59′E / 31.900°N 35.983°E / 31.900; 35.983Country JordanGovernorateAmman GovernorateTime zoneUTC + 2 Al-Rajib or simply Rajib is a town in Qweismeh area of Greater Amman Municipality in northwest Jordan.[1] It's known for the Cave of the Seven Sleepe…

VT Độixts ST T H B BT BB HS Đ Giành quyền tham dự 1 Iceland 10 7 1 2 16 7 +9 22 Vượt qua vòng loại vàoFIFA World Cup 2018 2 Croatia 10 6 2 2 15 4 +11 20 Giành quyền vào vòng 2 3 Ukraina 10 5 2 3 13 9 +4 17 4 Thổ Nhĩ Kỳ 10 4 3 3 14 13 +1 15 5 Phần Lan 10 2 3 5 9 13 −4 9 6 Kosovo 10 0 1 9 3 24 −21 1 Nguồn: FIFAQuy tắc xếp hạng: Các tiêu chí vòng loại Để chỉnh sửa các bảng xếp hạng: Bảng A, B, …

Christian radio network in the United States Rejoice RadioTypeRadio networkBrandingRejoice RadioCountryUnited StatesOwnerPensacola Christian College, Inc.[1]Launch dateDecember 1996[2]WebcastStreamingOfficial websiterejoice.org Rejoice Radio is a network of Christian radio stations airing a format of Christian talk and teaching and Christian music. The network is owned by Pensacola Christian College.[1] History Since 1971, Rejoice Radio has broadcast Christian music and p…