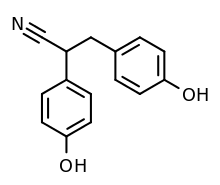
Chemical compound
Pharmaceutical compound
Diarylpropionitrile (DPN ), also known as 2,3-bis(p-hydroxyphenyl)propionitrile (2,3-BHPPN ), is a synthetic , nonsteroidal , and highly selective agonist of ERβ (IC50 = 15 nM)[ 1] scientific research to study the function of this receptor .[ 2] [ 3] ERα ,[ 4] affinity for GPER (GPR30) relative to estradiol .[ 5] antidepressant - and anxiolytic -like effects in animals via activation of the endogenous oxytocin system.[ 6] prinaberel (ERB-041, WAY-202041), WAY-200070 , and 8β-VE2 in 2004, ERB-196 (WAY-202196) in 2005, and certain phytoestrogens like liquiritigenin and nyasol (cis -hinokiresinol) since 2007.[ 7]
DPN is a racemic mixture of two enantiomers , (R)-DPN and (S)-DPN. Relative to (R)-DPN, (S)-DPN has between 3- and 7-fold higher affinity for ERβ and appears to have higher intrinsic activity in activating ERβ.[ 8] [ 9] [ 8] scientific research .[ 8]
See also
References
^ "2,3-Bis(4-hydroxyphenyl)propionitrile" . Sigmaaldrich.com . Retrieved 26 April 2022 .^ Pfaus JG, Jone LS, Flanagan-Cato LM, Blaustein JD (15 November 2014). "Female Sexual Behavior" . In Plant TM, Zeleznik AJ (eds.). Knobil and Neill's Physiology of Reproduction: Two-Volume Set . Academic Press. pp. 2311–. ISBN 978-0-12-397769-4 ^ ^ Hwang KA, Choi KC (5 November 2015). "Endocrine-Disrupting Chemicals with Estrogenicity Posing the Risk of Cancer Progression in Estrogen-Responsive Gene" . Advances in Molecular Toxicology . Academic Press. pp. 16–. ISBN 978-0-12-802430-0 ^ Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q (August 2010). "Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta" . Psychoneuroendocrinology . 35 (7): 1023– 33. doi :10.1016/j.psyneuen.2010.01.003 . PMC 2891004 PMID 20138435 . ^ Kudwa AE, McGivern RF, Handa RJ (April 2014). "Estrogen receptor β and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats" . Physiology & Behavior . 129 : 287– 296. doi :10.1016/j.physbeh.2014.03.004 . PMC 5802969 PMID 24631553 . ^ Deroo BJ, Buensuceso AV (September 2010). "Minireview: Estrogen receptor-beta: mechanistic insights from recent studies" . Molecular Endocrinology . 24 (9): 1703– 1714. doi :10.1210/me.2009-0288 PMC 5417404 PMID 20363876 . ^ a b c Carroll VM, Jeyakumar M, Carlson KE, Katzenellenbogen JA (January 2012). "Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands" . Journal of Medicinal Chemistry . 55 (1): 528– 537. doi :10.1021/jm201436k . PMC 3381613 PMID 22122563 . ^ Weiser MJ, Wu TJ, Handa RJ (April 2009). "Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress" . Endocrinology . 150 (4): 1817– 1825. doi :10.1210/en.2008-1355 . PMC 2659273 PMID 19074580 .
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown