|
Galidesivir
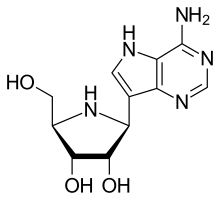
Galidesivir (BCX4430, immucillin-A) is an antiviral drug, an adenosine analog[1] (a type of nucleoside analog).[2] It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus.[3] Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.[4] It also shows broad-spectrum antiviral effectiveness against a range of other RNA virus families, including bunyaviruses, arenaviruses, paramyxoviruses, coronaviruses, flaviviruses, and phleboviruses.[5] Galidesivir has been demonstrated to protect against both Ebola and Marburg viruses in both rodents and monkeys, even when administered up to 48 hours after infection,[1] and development for use in humans was then being fast-tracked due to concerns about the lack of treatment options for the 2013-2016 Ebola virus epidemic in West Africa.[6] Galidesivir later showed efficacy against Zika virus in a mouse model.[7] Galidesivir abrogates viremia in Zika virus–infected rhesus Macaques.[8] Galidesivir is one of several antiviral drugs being tested for coronavirus disease 2019.[9] On April 9, 2020, BioCryst opened enrollment into a randomized, double-blind, placebo-controlled clinical trial to assess the safety, clinical impact and antiviral effects of galidesivir in patients with COVID-19.[4] See also
References
|
||||||||||||||||||||||||||||||