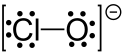Hypochlorite
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

P. RamleeInformasi latar belakangNama lahirTeuku Zakaria bin Teuku Nyak PutehNama lainP. RamleeLahir(1929-03-22)22 Maret 1929 Pulau Pinang, Straits SettlementsMeninggal29 Mei 1973(1973-05-29) (umur 44) Setapak, Kuala Lumpur, MalaysiaPekerjaanpelawak, pengusaha, dan aktorTahun aktif1945 - 1973 Suami/istri(Almh) Salmah Ismail (1961-1983) P. Ramlee atau nama sebenarnya Teuku Zakaria bin Teuku Nyak Puteh (22 Maret 1929 – 29 Mei 1973) adalah seorang aktor Malaysia pada tahun 1950…

The relationship between Baháʼí Faith and Native Americans has a history reaching back to the lifetime of ʻAbdu'l-Bahá and has multiplied its relationships across the Americas. Individuals have joined the religion and institutions have been founded to serve Native Americans and conversely have Native Americans serve on Baháʼí institutions. By 1963, Baháʼí sources claimed that members of some 83 tribes of Native Americans had joined the religion.[1] In North America diversifica…

Ivan Tričkovski bersama Makedonia di 2011Informasi pribadiNama lengkap Ivan TričkovskiTanggal lahir 18 April 1987 (umur 36)Tempat lahir Skopje, SFR YugoslaviaTinggi 1,82 m (5 ft 11+1⁄2 in)Posisi bermain Striker / Pemain sayapInformasi klubKlub saat ini APOELNomor 11Karier junior FK VardarKarier senior*Tahun Tim Tampil (Gol)2004-2006 FK Vardar 32 (6)2006-2007 FK Rabotnički 26 (14)2007–2010 Red Star Belgrade 25 (3)2009–2010 → Enosis Neon Paralimni (pinjaman) 29 (…

Brucella abortus PenyakitBrucella abortus infection (en) Pewarnaan GramGram-negatif TaksonomiSuperdomainBiotaDomainBacteriaKerajaanPseudomonadatiFilumPseudomonadotaKelasAlphaproteobacteriaOrdoRhizobialesFamiliBrucellaceaeGenusBrucellaSpesiesBrucella abortus lbs Brucella abortus adalah bakteri Gram-negatif pada genus Brucella yang merupakan salah satu penyebab bruselosis. Bakteri ini berbentuk batang, tidak berspora, tidak motil, dan aerobik.[1] Brucella terdiri atas 12 spesies, salah sat…

Gereja Santo Petrus RasulGereja Katolik Paroki Santo Petrus Rasul, PurwosariGereja Santo Petrus Rasul, Purwosari, SurakartaLokasiKota Surakarta, Jawa TengahNegaraIndonesiaDenominasiGereja Katolik RomaSejarahPendiriRomo Verhaar, SJArsitekturStatusGereja parokiStatus fungsionalAktifTipe arsitekturGerejaAdministrasiKeuskupanKeuskupan Agung Semarang Gereja Santo Petrus, yang bernama resmi Gereja Paroki Santo Petrus Rasul Purwosari atau lebih dikenal dengan nama Gereja Santo Petrus Purwosari atau Ger…

American politician For other people named Edwin Johnson, see Edwin Johnson (disambiguation). Ed Johnson26th and 34th Governor of ColoradoIn officeJanuary 11, 1955 – January 8, 1957LieutenantStephen McNicholsPreceded byDaniel I. J. ThorntonSucceeded byStephen McNicholsIn officeJanuary 10, 1933 – January 1, 1937LieutenantRay Herbert TalbotPreceded byBilly AdamsSucceeded byRay Herbert TalbotUnited States Senatorfrom ColoradoIn officeJanuary 3, 1937 – January 3, 195…

سالي آن هاوز (بالإنجليزية: Sally Ann Howes) معلومات شخصية الميلاد 20 يوليو 1930 [1] سانت جونز وود [لغات أخرى] الوفاة 19 ديسمبر 2021 (91 سنة) [2] بالم بيتش غاردنز[2] مواطنة المملكة المتحدة الزوج ريتشارد أدلر (1958–1966) الأب بوبي هاوز [لغات أخرى]…

This is a list of stadiums that currently serve as the home venue for NCAA Division I college baseball teams. Conference affiliations reflect those in the upcoming 2024 NCAA baseball season. In addition, venues which are not located on campus or are used infrequently during the season have been listed. Among Division I conferences that sponsor men's and women's basketball, the Big Sky Conference and Mid-Eastern Athletic Conference are the only ones that do not sponsor baseball. Current stadiums …

Constituency of Bangladesh's Jatiya Sangsad Pabna-4Constituencyfor the Jatiya SangsadDistrictPabna DistrictDivisionRajshahi DivisionElectorate362,483 (2018)[1]Current constituencyCreated1973PartyAwami LeagueMember(s)Galibur Rahman Sharif Pabna-4 is a constituency represented in the Jatiya Sangsad (National Parliament) of Bangladesh since 2024 by Galibur Rahman Sharif of the Awami League. Boundaries The constituency encompasses Atgharia and Ishwardi upazilas.[2][3] History…

Small nucleolar RNA U2-30Predicted secondary structure and sequence conservation of snoU2-30IdentifiersSymbolsnoU2-30RfamRF00493Other dataRNA typeGene; snRNA; snoRNA; CD-boxDomain(s)EukaryotaGOGO:0006396 GO:0005730SOSO:0000593PDB structuresPDBe In molecular biology, Small nucleolar RNA U2-30 is a non-coding RNA (ncRNA) molecule which functions in the modification of other small nuclear RNAs (snRNAs). This type of modifying RNA is usually located in the nucleolus of the eukaryotic cell which is a…

Le ministère des Affaires étrangères de la République de Serbie Kneza Miloša (en serbe cyrillique : Кнеза Милоша) est une rue de Belgrade, la capitale de la Serbie. Elle est située dans la municipalité de Savski venac. La rue est ainsi nommée en hommage au prince (en serbe : knez) Miloš Obrenović (1780-1860), qui fut le chef du Second soulèvement serbe contre les Turcs (1815). Localisation La rue Kneza Miloša commence au carrefour de la Place Nikola Pašić, du B…

Voce principale: CSI - Scena del crimine. La tredicesima stagione della serie televisiva CSI - Scena del crimine è stata trasmessa sul canale statunitense CBS dal 26 settembre 2012 al 15 maggio 2013.Invece in Italia, la prima parte della stagione (ep. 1-13) è stata trasmessa dal 14 marzo al 30 maggio 2013 e la seconda parte (ep. 14-22) dal 13 settembre al 1º novembre 2013 su Fox Crime. Il tredicesimo episodio, In vino veritas, costituisce la prima parte di un crossover con CSI: NY, che proseg…

Museo del papiro Corrado BasileCortile del museo. UbicazioneStato Italia LocalitàSiracusa IndirizzoVia Nizza 14, 96100 Siracusa Coordinate37°03′37.79″N 15°17′53.35″E / 37.060498°N 15.298152°E37.060498; 15.298152Coordinate: 37°03′37.79″N 15°17′53.35″E / 37.060498°N 15.298152°E37.060498; 15.298152 CaratteristicheTipoCultura del papiro Istituzione1987 FondatoriCorrado Basile e Anna Di Natale Apertura30 settembre 1987 Visitatori8 083…

American contemporary Christian singer Greg LongBackground informationBirth nameGregory Alan LongBorn (1966-12-12) December 12, 1966 (age 57)OriginAberdeen, South Dakota, USGenresContemporary Christian musicOccupation(s)Musician, songwriterInstrument(s)VocalsYears active1994–presentLabelsMyrrh/Pakaderm, Word, ChristianMusical artist Greg Long (born December 12, 1966) is an American contemporary Christian music solo artist[1][2] and also a member of the contemporary Christi…

SchoolLycée Français Pierre Loti d'IstanbulAddressTarabya Campus: Haydar Aliyev Caddesi n°128Beyoğlu Campus: Tomtom Kaptan Sok. BeyoğluInformationPrincipalFrédéric ColombelWebsitepierreloti.k12.tr Lycée Français Pierre Loti d'Istanbul is an international French school located in Istanbul. It was formerly known as Papillon and later took its name from the French writer Pierre Loti, who lived in Istanbul for a period of time. The school provides education from preschool to the final year …

Pour les articles homonymes, voir Smog (homonymie). Smog à New York en 1978. Vues de Pékin un jour après la pluie (à gauche) et un jour ensoleillé avec le smog (à droite). Smog à Kuala Lumpur en 2005. Le smog[1], fumard[1] ou brumée[1] est un brouillard grisâtre urbain qui limite la visibilité dans l’atmosphère. Issu du mélange de particules fines et d'ozone, le smog est associé à plusieurs effets néfastes pour la santé et pour l'environnement. Étymologie Le terme smog est un …

Japanese commercial CubeSat WE WISHA collection of CubeSats at Tsukuba Space Center prior to their launch in 2012, with WE WISH visible on the far leftMission typeTechnology demonstrationAmateur radioEarth observationOperatorMeisei Amateur Radio ClubCOSPAR ID2012-038F (1998-067CS)SATCAT no.38856Mission duration158 days (achieved)100 days (planned) Spacecraft propertiesSpacecraft typeCubeSatBusCubeSatManufacturerMeisei ElectricMeisei Amateur Radio ClubLaunch mass1 kg (2.2 lb)Dimensions1…

Chronologies Données clés 1866 1867 1868 1869 1870 1871 1872Décennies :1830 1840 1850 1860 1870 1880 1890Siècles :XVIIe XVIIIe XIXe XXe XXIeMillénaires :-Ier Ier IIe IIIe Chronologies géographiques Afrique Afrique du Sud, Algérie, Angola, Bénin, Botswana, Burkina Faso, Burundi, Cameroun, Cap-Vert, République centrafricaine, Comores, République du Congo, République démocratique du Congo, Côte d'Ivoire, Djibouti, Égypte, …

Nico Touches the Walls discographyNICO Touches the Walls performing in 2010Studio albums8Compilation albums1Video albums7Music videos26EPs5Singles27Promotional singles7 The discography of the Japanese band Nico Touches the Walls consists of eight studio albums, 27 extended plays, eleven singles, seven promotional singles, seven video albums, and 26 music videos.[1] Nico Touches the Walls (stylized as NICO Touches the Walls) was a Japanese rock band formed in 2004. In the same year, they …

Tigre de Longdan Panthera zdanskyi Crâne holotype. 2.55–2.16 Ma PreꞒ Ꞓ O S D C P T J K Pg N ↓ Pléistocène inférieur.Classification Règne Animalia Embranchement Chordata Classe Mammalia Infra-classe Placentalia Ordre Carnivora Sous-ordre Feliformia Famille Felidae Sous-famille Pantherinae Genre Panthera Espèce† Panthera zdanskyiMazák (d), Christiansen (d) & Kitchener (d), 2011 Panthera zdanskyi, le Tigre de Longdan[1], est une espèce éteinte de …