Pinnick oxidation
| |||||||||
Read other articles:

This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (February 2024) The Mata Bhimeshwari Devi Temple in Beri, Haryana, India is a popular religious destination. Fair It holds a large fair twice a year, where lakhs of devotees come to worship the goddess. The temple is decorated with hundreds of shops, and newly married couples tie their nuptial knots before the goddess. The Mundan ceremony …

Lambang Wangsa Dreux Jean II (1265–1309), disebut Jean yang baik, merupakan Comte Dreux dan Braine, ia adalah putra Robert IV dari Dreux dan Beatrice, Comtesse Montfort. Robert bertempur dengan Philippe IV dari Prancis dalam perangnya di Flandria, termasuk pengepungan Veurne, Cassel, de Bergues dan Lille pada 1297. Ia berada di Pertempuran Courtrai (berlangsung di dekat Cambrai), di mana pasukan Prancis di bawah pimpinan Robert II dari Artois menderita kekalahan tak terduga. Pada 1304, ia bert…

Koordinat: 34°54′S 138°36′E / 34.9°S 138.6°E / -34.9; 138.6 Rymill Park / Murlawirrapurka (Park 14), tempat populer di Park Lands Ladang bunga di Adelaide Park Lands Eukaliptus di South Park Lands Adelaide Park Lands adalah taman berbentuk angka delapan yang dibelah oleh Sungai Torrens. Taman ini terletak di antara Hackney dan Thebarton, memisahkan City of Adelaide dengan kota-kota pinggiran yang membentuk wilayah metropolitan Adelaide, ibu kota Australia Selatan.…

Song by Rudy Toombs One Scotch, One Bourbon, One BeerSingle by Amos MilburnB-sideWhat Can I Do?ReleasedAugust 1953 (1953-08)RecordedJune 30, 1953StudioAudio-Video Recording, New York CityGenreBluesLength2:50LabelAladdinSongwriter(s)Rudy ToombsAmos Milburn singles chronology Let Me Go Home Whiskey (1953) One Scotch, One Bourbon, One Beer (1953) Good Good Whiskey (1953) One Bourbon, One Scotch, One Beer (originally One Scotch, One Bourbon, One Beer) is a blues song written by Rudy Toombs…

Japanese educational television channel You can help expand this article with text translated from the corresponding article in Japanese. (December 2009) Click [show] for important translation instructions. View a machine-translated version of the Japanese article. Machine translation, like DeepL or Google Translate, is a useful starting point for translations, but translators must revise errors as necessary and confirm that the translation is accurate, rather than simply copy-pasting machi…

Artikel ini bukan mengenai Isaac Hays. Untuk kegunaan lain, lihat Isaac Hayes (disambiguasi). Isaac HayesHayes pentas pada Juni 2007LahirIsaac Lee Hayes Jr.(1942-08-20)20 Agustus 1942Covington, Tennessee, Amerika SerikatMeninggal10 Agustus 2008(2008-08-10) (umur 65)Memphis, Tennessee, Amerika SerikatMakamMemorial Park Cemetery, Memphis, Tennessee, Amerika SerikatPekerjaan Penyanyi penulis lagu pemeran produser Tahun aktif1963–2008Suami/istri Emily Ruth Watson (…

Lithograph[1] on a plate produced by Iroquois China Company from 1930s or 1940s Restaurant ware, or most commonly hotelware,[2][3][4][5][6][7][8] is vitrified, ceramic tableware which exhibits high mechanical strength and is produced for use in hotels and restaurants.[9] Tableware used in railway dining cars, passenger ships and airlines are also included in this category. Collectable hotelware was usually made of stoneware …

Italian actor This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (January 2013) (Learn how and when to remove this message) Ninetto DavoliDavoli in 2014 in VeniceBorn (1948-10-11) 11 October 1948 (age 75)San Pietro a Maida, Calabria, ItalyOccupationActorYears active1964–presentHeight1.74 m (5 ft 9 in) Giovanni Ninetto Davoli (born 11 Octob…

Lineage of Theravada Buddhist monasticism For the meditation practices called Kammaṭṭhāna (Place of work), see Kammaṭṭhāna. This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages) This article uses bare URLs, which are uninformative and vulnerable to link rot. Please consider converting them to full citations to ensure the article remains verifiable and maintains a consistent citation styl…

Este artículo o sección necesita referencias que aparezcan en una publicación acreditada. Busca fuentes: «The Lovely Bones (película)» – noticias · libros · académico · imágenesEste aviso fue puesto el 27 de noviembre de 2012. The Lovely BonesTítulo Desde mi cielo (Hispanoamérica)Ficha técnicaDirección Peter JacksonProducción Peter JacksonFran WalshCarolynne CunninghamAimée PeyronnetGuion Peter JacksonFran WalshPhilippa BoyensBasada en Desde mi cielo de Alic…

Men's cycling road raceat the Games of the XXI OlympiadBernt JohanssonVenueMontréal, CanadaDate26 July 1976Competitors134 from 40 nationsWinning time4:46:52Medalists Bernt Johansson Sweden Giuseppe Martinelli Italy Mieczysław Nowicki Poland← 19721980 → Cycling at the1976 Summer OlympicsRoad cyclingIndividual road racemenTeam time trialmenTrack cyclingTrack time trialmenIndividual pursuitmenTeam pursuitmenSprintmenvte The men's individual road race…

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「弐」…

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「弐」…

Filipino corned beef fritter Tortang carne norteAlternative namesCorned beef omelette, carne norte omelet, tortang corned beefCourseMain course, side dishPlace of originPhilippinesServing temperatureWarmMain ingredientsCorned beef, eggsSimilar dishesTortang giniling, Tortang sardinas Tortang carne norte, also known as corned beef omelette, is an omelette or fritter from Filipino cuisine made by pan-frying an egg and shredded canned corned beef (carne norte) mixture. It is usually seasoned with s…

Niedersoultzbachcomune Niedersoultzbach – Veduta LocalizzazioneStato Francia RegioneGrand Est Dipartimento Basso Reno ArrondissementSaverne CantoneIngwiller TerritorioCoordinate48°51′N 7°28′E / 48.85°N 7.466667°E48.85; 7.466667 (Niedersoultzbach)Coordinate: 48°51′N 7°28′E / 48.85°N 7.466667°E48.85; 7.466667 (Niedersoultzbach) Altitudine188-233 m s.l.m. Superficie4,22 km² Abitanti259[1] (2009) Densità61,37 ab…

Olympic gymnastics event Men's ringsat the Games of the X OlympiadOlympic Games at the Los Angeles Memorial ColiseumVenueLos Angeles Memorial ColiseumDate12 AugustCompetitors14 from 6 nationsWinning score56.9Medalists George Gulack United States Bill Denton United States Giovanni Lattuada Italy← 19281936 → Gymnastics at the1932 Summer OlympicsAll-aroundmenTeammenFloormenHorizontal barmenIndian clubsmenParallel barsmenPommel horsemenRingsmenRope clim…

Economic and wage policy model Part of a series onSocial democracy History Age of Enlightenment Frankfurt Declaration French Revolution Godesberg Program Humanism Internationalist–defencist schism Keynesianism Labor movement Marxism Orthodox Revisionist Nordic model Reformist–revolutionary dispute Socialism Revolutions of 1848 Utopian socialism Welfare capitalism Concepts Civil liberties Critical theory Democracy Economic Industrial Representative Dirigisme Environmentalism Environmental pro…
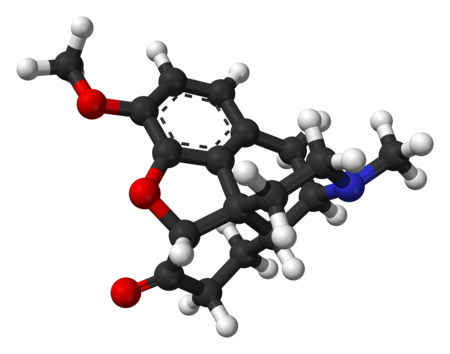
Le informazioni riportate non sono consigli medici e potrebbero non essere accurate. I contenuti hanno solo fine illustrativo e non sostituiscono il parere medico: leggi le avvertenze. Idrocodone Nome IUPAC(5R,9R,13S,14R)-3-metossi-17 -metil-4,5-epossimorfinan-6-one Caratteristiche generaliFormula bruta o molecolareC18H21NO3 Massa molecolare (u)299,368 Numero CAS125-29-1 Numero EINECS204-733-9 Codice ATCR05DA03 PubChem5284569 DrugBankDB00956 SMILESCN1CCC23C4C1CC5=C2C(=C(C=C5)OC)OC3C(=O)CC4 Propr…

Allied aerial bombing raids in Germany Bombing of HamburgPart of strategic bombing during World War IIAftermath in the Eilbek district of Hamburg. This picture was taken after much of the rubble had been cleared, probably after VE day.DateSeptember 10, 1939– April 14, 1945LocationHamburg, Germany53°33′3″N 9°59′37″E / 53.55083°N 9.99361°E / 53.55083; 9.99361Result Allied victoryBelligerents United Kingdom United States Nazi GermanyCasualties and l…

Upper house of the Alabama legislature This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Alabama Senate – news · newspapers · books · scholar · JSTOR (April 2014) (Learn how and when to remove this message) 32°22′36″N 86°17′56″W / 32.37667°N 86.29889°W / 32.37667; -86.29889 Ala…




