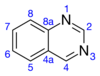
Quinazoline
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Cari artikel bahasa Cari berdasarkan kode ISO 639 (Uji coba) Cari berdasarkan nilai Glottolog Kolom pencarian ini hanya didukung oleh beberapa antarmuka Halaman rumpun acak Rumpun bahasaArab Semenanjung Bahasa Arab JazirahBahasa Arab SelatanPersebaranSemenanjung ArabPenggolongan bahasaAfroasiatikSemitSemit TengahArabArab SemenanjungKode bahasaGlottologarab1393 Portal BahasaSunting kotak info • L • B • PWBantuan penggunaan templat ini Gambaran keselu…

William Rehnquist 16º Presidente della Corte Suprema degli Stati UnitiDurata mandato26 settembre 1986 –3 settembre 2005 PredecessoreWarren Earl Burger SuccessoreJohn G. Roberts Dati generaliPartito politicoRepubblicano Firma William Hubbs Rehnquist (Milwaukee, 1º ottobre 1924 – Arlington, 3 settembre 2005) è stato un giurista statunitense. Fu giudice associato della Corte Suprema degli Stati Uniti dal 1972 e il suo 16º Giudice Capo dal 1986 fino alla sua morte. Durante…

Le informazioni riportate non sono consigli medici e potrebbero non essere accurate. I contenuti hanno solo fine illustrativo e non sostituiscono il parere medico: leggi le avvertenze. Funzioni immunitarie di leucociti I leucociti (dal greco λευκός, leukós, «bianco» e κύτος, cýtos, «cellula»), globuli bianchi o WBC (dall'inglese white blood cells), sono cellule mature della porzione corpuscolata del sangue. Essi sono presenti nella maggior parte dei distretti tissutali del corpo…

Disambiguazione – Se stai cercando il filosofo che dà il nome al dialogo platonico, vedi Fedone di Elide. FedoneTitolo originaleΦαίδων Altri titoliSull'anima D.N. Chodowiecki, Morte di Socrate (XVIII-XIX secolo) AutorePlatone 1ª ed. originaleIV secolo a.C. Generedialogo Sottogenerefilosofico Lingua originalegreco antico PersonaggiSocrate, Fedone, Echecrate, Critone, Simmia, Cebète, il messo degli Undici, il carceriere, vari altri discepoli socratici SerieDialoghi platonici, I tet…

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Kommando Landstreitkraefte – berita · surat kabar · buku · cendekiawan · JSTOR (January 2013) Kommando LaSK— XXX —Bendera Komando LandstreitkräfteAktif1 Desember 1972 — 2 Oktober 1990Negara Jerman Ti…

Mikhaēl III Mikhaēl III (Yunani: Μιχαήλ; Januari 840 – 24 September 867) adalah Kaisar Romawi Timur dari 842 hingga 867. Mikhaēl III adalah penguasa ketiga dan dianggap sebagai anggota terakhir dinasti Amoria (atau Frigia). Dia dijuluki si Pemabuk (ὁ Μέθυσος) oleh para sejarawan dari Dinasti Makedonia, tetapi penelitian sejarah modern merehabilitasi reputasinya hingga batas tertentu, menunjukkan peran penting yang dimainkan pemerintahannya dalam kebangkitan kekuasaan Bizantiu…
Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Februari 2023. Rolf WankaLahir(1901-02-14)14 Februari 1901Wina, AustriaMeninggal28 November 1982(1982-11-28) (umur 81)Munich, JermanPekerjaanPemeranTahun aktif1931–1976Tanda tangan Rolf Wanka (14 Februari 1901 – 28 November 1982) adalah seor…

Kamen Rider GotchardGenreTokusatsuSuperhero fictionScience fictionBerdasarkanKonsep Kamen Rideroleh Shotaro IshinomoriPengembangIshimori ProductionsToei CompanyDitulis olehKeiichi HasegawaHiroki UchidaSutradaraRyuta TasakiPemeranJunsei MotojimaReiyo MatsumotoYasunari FujibayashiOto AbeRikiya TomizonoRikuto KumakiItono OkitaKanon MiyaharaArisa SakamakiAmon KabeSaki FukudaKenta KamakariYoko MinaminoKanji IshimaruPengisi suaraMisato FukuenNobuyuki HiyamaNaratorKatsuyuki KonishiLagu pembukaCHEMY×ST…

PerdizioneMiriam HopkinsTitolo originaleThe Story of Temple Drake Lingua originaleinglese Paese di produzioneStati Uniti d'America Anno1933 Durata70 min Dati tecniciB/Nrapporto: 1,37:1 Generedrammatico RegiaStephen Roberts Soggettodal romanzo Santuario di William Faulkner SceneggiaturaOliver H.P. Garrett Maurine Dallas Watkins (non accreditata) ProduttoreBenjamin Glazer Casa di produzioneParamount Pictures FotografiaKarl Struss MusicheKarl Hajos, Bernhard Kaun (non accreditati) John Leipold, Ral…

Arthur AsheArthur Ashe, winning the 1975 ABN World Tennis Tournament in RotterdamKebangsaan Amerika SerikatLahir(1943-07-10)10 Juli 1943Richmond, VirginiaMeninggal6 Februari 1993(1993-02-06) (umur 49)New York City, New YorkTinggi6 ft 1 in (185 cm)Memulai pro1969 (tur amatir dari 1959)Pensiun1980Tipe pemainTangan kanan (one-handed backhand)Total hadiah$1,584,909 (ATP)Int. Tennis HoF1985 (member page)TunggalRekor (M–K)1085–337 (76.3%) in pre Open-Era & Open Era[…

Carrie UnderwoodCarrie Underwood di American Music Award pada tahun 2018Informasi latar belakangNama lahirCarrie Marie UnderwoodLahir10 Maret 1983 (umur 41)Muskogee, Oklahoma, Amerika SerikatAsalChecotah, Oklahoma, Amerika SerikatGenre Country Pop Pekerjaan Penyanyi Penulis lagu Instrumen Vokal Gjtar Piano Tahun aktif2005-kiniLabelArista Nashville, Capitol NashvilleArtis terkaitGretchen WilsonSitus webwww.carrieunderwoodofficial.com Carrie Marie Underwood (lahir 10 Maret 1983) merupakan pen…

ATP Jenis Pesawat penumpang Pembuat British Aerospace Penerbangan perdana 6 August 1986 Diperkenalkan 1988 Pengguna utama West Air Sweden Dibuat 1988–1996 Jumlah 64 Dikembangkan dari Hawker Siddeley HS 748 British Aerospace ATP adalah sebuah pesawat penumpang yang diproduksi oleh British Aerospace, dirancang sebagai evolusi dari Hawker Siddeley HS 748. Krisi bahan bakar dan meningkatnya kekhawatiran akan tingkat kebisingan pesawat membuat perencana bisnis di British Aerospace percaya bahw…

Katherine TaiTai pada Desember 2018 Perwakilan Dagang Amerika SerikatMulai menjabat18 Maret 2021PresidenJoe Biden Informasi pribadiLahir1974/1975 (umur 48–49)[1]Connecticut, Amerika SerikatPartai politikPartai DemokratPendidikanUniversitas Yale (BA)Universitas Harvard (JD)Sunting kotak info • L • B Katherine Chi Tai adalah mantan anggota Jaksa Federal dan Ahli Hukum Ekonomi, yang menjabat sebagai kepala penasehat dagang untuk United States House Committee on Ways and…

Gagang-bayam hitam Himantopus novaezelandiae Wild kakī in Lake TekapoStatus konservasiTerancam kritisIUCN22693690 TaksonomiKerajaanAnimaliaFilumChordataKelasAvesOrdoCharadriiformesFamiliRecurvirostridaeGenusHimantopusSpesiesHimantopus novaezelandiae Gould, 1841 Distribusi EndemikSelandia Baru lbs Gagang-bayam hitam ( Himantopus novaezelandiae ) atau kakī ( Māori ) adalah burung perandai yang ditemukan di Selandia Baru. Ini adalah salah satu burung paling langka di dunia, dengan 169 burung dew…

Hotel and casino in Paradise, Nevada Luxor Hotel redirects here. For the New York City hotel, see Luxor Hotel (New York City). Luxor Las VegasShow map of Las Vegas StripShow map of Nevada Location Paradise, Nevada, U.S. Address 3900 South Las Vegas BoulevardOpening dateOctober 15, 1993; 30 years ago (October 15, 1993)ThemeAncient EgyptNo. of rooms4,407Total gaming space65,214 sq ft (6,058.6 m2)Permanent showsAmerica's Got Talent Presents Superstars LiveBlue Man GroupC…

artikel ini perlu dirapikan agar memenuhi standar Wikipedia. Tidak ada alasan yang diberikan. Silakan kembangkan artikel ini semampu Anda. Merapikan artikel dapat dilakukan dengan wikifikasi atau membagi artikel ke paragraf-paragraf. Jika sudah dirapikan, silakan hapus templat ini. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Bulimia nervosa adalah kelainan cara makan yang terlihat dari kebiasaan makan berlebihan yang terjadi secara terus menerus. Bulimia sering terjadi pa…

Basilika Santo Paulinus TrierKoordinat: 49°45′44.9″N 6°39′7.88″E / 49.762472°N 6.6521889°E / 49.762472; 6.6521889LokasiTrierNegaraJermanDenominasiKatolik RomaSejarahDidirikanabad ke-4DedikasiPaulinus dari TrierArsitekturStatusBasilika (minor)Status fungsionalactiveArsitekBalthasar NeumannGayaBarokPeletakan batu pertama1734Selesai1753 Menara lonceng Santo Paulinus dengan prasasti Salib Besi di sebelah kiri untuk mengenang kemartiran Legiun Thebes Basilika Santo…

Jembatan MerahDesaNegara IndonesiaProvinsiKalimantan SelatanKabupatenHulu Sungai SelatanKecamatanPadang BatungKode pos71281Kode Kemendagri63.06.02.2009 Luas... km²Jumlah penduduk... jiwaKepadatan... jiwa/km² Jembatan Merah adalah salah satu desa di wilayah kecamatan Padang Batung, Kabupaten Hulu Sungai Selatan, Provinsi Kalimantan Selatan, Indonesia. Pranala luar (Indonesia) Keputusan Menteri Dalam Negeri Nomor 050-145 Tahun 2022 tentang Pemberian dan Pemutakhiran Kode, Data Wilayah Admin…

Attilio Lombardo Informasi pribadiTanggal lahir 6 Januari 1966 (umur 58)Tempat lahir Santa Maria la Fossa, ItaliaTinggi 1,77 m (5 ft 9+1⁄2 in)Posisi bermain gelandang/sisi kananKarier senior*Tahun Tim Tampil (Gol)1983–1985 Pergocrema 38 (9)1985–1989 Cremonese 141 (17)1989–1995 Sampdoria 201 (34)1995–1997 Juventus 35 (2)1997–1999 Crystal Palace 43 (8)1999–2001 Lazio 33 (2)2001–2002 Sampdoria 34 (4)Tim nasional‡1990–1997 Italia 19 (3)Kepelatihan1998 Crys…

Azriel HildesheimerFoto Asriel Hildesheimer.Lahir(1820-05-11)11 Mei 1820Halberstadt, Saxony, PrusiaMeninggal12 Juli 1899(1899-07-12) (umur 79)Berlin, Prusia, JermanKebangsaanJermanSuami/istriHenriette HirschAnakHirsch Hildesheimer [de] Azriel Hildesheimer (juga Esriel dan Israel, bahasa Yiddi: עזריאל הילדעסהיימער; 11 Mei 1820 – 12 Juli 1899)[1] adalah seorang rabi dan pemimpin Yahudi Ortodoks asal Jerman. Ia dianggap sebagai pionir …