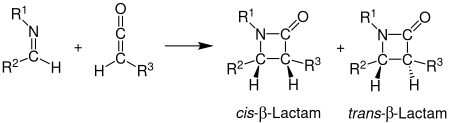
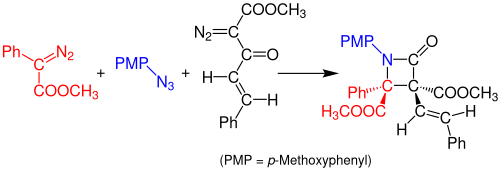
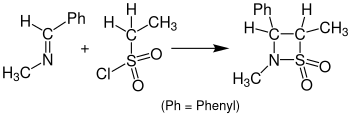
Staudinger synthesis
|
Read other articles:

Questa voce o sezione sugli argomenti India e vessillologia non cita le fonti necessarie o quelle presenti sono insufficienti. Commento: Vce senza fonti Puoi migliorare questa voce aggiungendo citazioni da fonti attendibili secondo le linee guida sull'uso delle fonti. Segui i suggerimenti del progetto di riferimento. भारत का ध्वजFlag of IndiaSoprannomeतिरंगा (Tiranga) Proporzioni2:3 Simbolo FIAV ColoriRGB (R:255 G:153 B:51) …

Grindr Tipeaplikasi seluler, online dating service (en) dan Perusahaan publik Versi pertama25 Maret 2009 (2009-03-25)Versi stabilAndroid: 6.17.0 iOS: 6.18.0 Android: 8.12.1 GenreJejaring geososialLisensiLisensi proprietarium Karakteristik teknisSistem operasiApple iOSAndroidBlackBerry OSInformasi pengembangPembuatJoel Simkhai (en) PengembangGrindr LLCInformasi tambahanSitus webGrindr.comSubredditgrindr Sunting di Wikidata • L • B • Bantuan penggunaan templat ini Grindr …

Ini adalah nama Tionghoa; marganya adalah Chang. Angela ChangChang pada 2009.Lahir19 Januari 1982 (umur 42)Zhongli, Taoyuan, TaiwanKebangsaanTaiwanAlmamaterSir Winston Churchill Secondary SchoolPekerjaanPenyanyi, pemeran, pemandu acaraTahun aktif2000–sekarangKeluargaConnie Chang Angela Chang Hanzi tradisional: 張韶涵 Hanzi sederhana: 张韶涵 Alih aksara Mandarin - Hanyu Pinyin: Zhāng Shàohán Karier musikGenreMandopopInstrumenVokalLabelLinfair RecordsSony Music EntertainmentWo…

artikel ini perlu dirapikan agar memenuhi standar Wikipedia. Tidak ada alasan yang diberikan. Silakan kembangkan artikel ini semampu Anda. Merapikan artikel dapat dilakukan dengan wikifikasi atau membagi artikel ke paragraf-paragraf. Jika sudah dirapikan, silakan hapus templat ini. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) iPhone 6S and iPhone 6S PlusiPhone 6S in Rose GoldMerekApple Inc.Seri9thJaringanGSM, CDMA, 3G, EVDO, HSPA+, LTE/4G, LTE Advanced/4G+Rilis pertama25 S…

Artikel ini memiliki beberapa masalah. Tolong bantu memperbaikinya atau diskusikan masalah-masalah ini di halaman pembicaraannya. (Pelajari bagaimana dan kapan saat yang tepat untuk menghapus templat pesan ini) Artikel ini mungkin mengandung riset asli. Anda dapat membantu memperbaikinya dengan memastikan pernyataan yang dibuat dan menambahkan referensi. Pernyataan yang berpangku pada riset asli harus dihapus. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Artikel ini membut…

Artikel ini membutuhkan penyuntingan lebih lanjut mengenai tata bahasa, gaya penulisan, hubungan antarparagraf, nada penulisan, atau ejaan. Anda dapat membantu untuk menyuntingnya. Universitas PancasilaLambang Universitas PancasilaJenisPerguruan tinggi swastaDidirikan28 Oktober 1966[1]RektorProf. Dr. Sri Widyastuti (Plt.)LokasiDKI Jakarta, DKI Jakarta, IndonesiaKampusUrbanWarnaBiruNama julukanJaket BiruSitus webhttp://www.univpancasila.ac.id Universitas Pancasila (UP) merupakan salah sat…

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Penaklukan Hispania oleh Umayyah – berita · surat kabar · buku · cendekiawan · JSTOR lbsPenaklukan Umayyah di Hispania Guadalete Toulouse Covadonga Tours Penaklukan Hispania oleh Umayyah (711–718) dimulai…

Bandar Udara Ayers RockBandar Udara ConnellanBandar Udara Ayers Rock/ConnellanIATA: AYQICAO: YAYEInformasiJenisPublicPengelolaVoyages Indigenous Tourism Australia Pty LtdLokasiUluruKetinggian dpl mdplKoordinat25°11′10″S 130°58′32″E / 25.18611°S 130.97556°E / -25.18611; 130.97556Koordinat: 25°11′10″S 130°58′32″E / 25.18611°S 130.97556°E / -25.18611; 130.97556PetaYAYELocation in the Northern TerritoryLandasan pacu Ar…

Form of censorship involving religious authority The Buddhas of Bamiyan were destroyed by the Taliban in 2001. Freedom of religion Concepts Laicism Religious discrimination Religious censorship Religious liberty Religious pluralism Secularism Separation of church and state Anti-clericalism School prayer Catholic priests in public office Confessionalism Theocracy State religion Secular state Confessional state Atheist state Status by country Africa Algeria Angola Benin Botswana Burkina Faso Burun…
Species of bat Dusky pipistrelle Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Chiroptera Family: Vespertilionidae Genus: Pipistrellus Species: P. hesperidus Binomial name Pipistrellus hesperidus(Temminck, 1840) The dusky pipistrelle (Pipistrellus hesperidus) is a small pipistrelle bat found in Africa.[2] References ^ Piraccini, R. (2016). Pipistrellus hesperidus. Th…

American politician Tricia Farley-BouvierMember of the Massachusetts House of Representatives from the 2nd Berkshire DistrictIncumbentAssumed office 2011Preceded byChristopher Speranzo Personal detailsBornPittsfield, MassachusettsPolitical partyDemocraticResidencePittsfield, MassachusettsAlma materSalve Regina University, 1986University of Connecticut, 1991OccupationLegislatorTeacherWebsitehttp://triciafarleybouvier.com Tricia Farley-Bouvier is an American state legislator serving in the Mas…

Jon McLaughlin Nazionalità Scozia Altezza 191 cm Peso 83 kg Calcio Ruolo Portiere Squadra Rangers Carriera Giovanili Harrogate Town Squadre di club1 2007-2008 Harrogate Town21 (-?)2008-2014 Bradford City125 (-147)[1]2014-2017 Burton Albion133 (-129)2017-2018 Hearts33 (-32)2018-2020 Sunderland78 (-84)[2]2020- Rangers29 (-19) Nazionale 2018-2019 Scozia2 (-1) 1 I due numeri indicano le presenze e le reti segnate, per le sole partite d…

Синелобый амазон Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:ЗавропсидыКласс:Птиц�…

Halaman ini memuat daftar lagu yang ditayangkan di Victorious Episode Judul Dinyanyikan oleh Rilis secara Digital Musim Soundtrack Pilot Make It Shine Tori Vega Ya 1 Victorious The Birthweek Song You're the Reason Tori Vega Ya 1 Victorious Jade Dumps Beck Chicago Trina Vega Tidak 1 N/A Tori the Zombie Finally Falling Tori Vega dan Beck Oliver* Ya 1 Victorious Survival of the Hottest Make It Shine Victorious Cast Tidak 1 N/A Wi-Fi in the Sky You're the Reason Trina Vega Tidak 1 N/A The Great Ping…

Writing system used for the Mongolian language This article is about the original Mongolian writing system. For later developments, see Mongolian writing systems. For the language, see Mongolian language. Mongolian scriptᠮᠣᠩᠭᠣᠯ ᠪᠢᠴᠢᠭPoem composed and brush-written by Injinash, 19th centuryScript type Alphabet CreatorTata-tongaTime periodc. 1204 – 1941 (used as main script)1941 – Present (used as co script)DirectionVertical up-to-down, left-to-rightLanguagesMongoli…

Christmas song by Paul McCartney Wonderful Christmas Time redirects here. For Diana Ross' 2018 Christmas album reissue, see A Very Special Season. Wonderful ChristmastimeSingle by Paul McCartneyB-sideRudolph the Red-Nosed Reggae (Instrumental)Released16 November 1979 (1979-11-16)Recorded30 August 1979StudioLower Gate Farm (Sussex)Genre Christmas synth-pop Length3:45Label Parlophone Columbia Songwriter(s)Paul McCartneyProducer(s)Paul McCartneyPaul McCartney singles chronology Eat a…

Japanese manga series B't XFirst tankōbon volume cover, as published by Tokyopopビート・エックス(Bīto Ekkusu)GenreAdventure[1]Science fiction[1] MangaWritten byMasami KurumadaPublished byKadokawa ShotenEnglish publisherNA: TokyopopMagazineShōnen AceDemographicShōnenOriginal runOctober 1994 – January 2000Volumes16 (List of volumes) Light novelWritten bySukehiro TomitaIllustrated byMasami KurumadaPublished byKadokawa ShotenImprintNewtype Novel…

Australian politician Not to be confused with John Murray (sheep breeder). John MurrayJohn Murray, May 1901Member of the Queensland Legislative Assemblyfor NormanbyIn office19 May 1888 – 1 March 1901Preceded byJohn StevensonSucceeded byGeorge FoxMember of the Queensland Legislative CouncilIn office12 March 1901 – 13 November 1903 Personal detailsBornJohn Murray(1837-08-15)15 August 1837Mauchline, Ayrshire, ScotlandDied18 November 1917(1917-11-18) (aged 80)Longreach, Qu…

Pour les articles homonymes, voir Giraud. Joël Giraud Joël Giraud en 2017. Fonctions Député français En fonction depuis le 21 juin 2022(1 an, 10 mois et 12 jours) Réélection 19 juin 2022 Circonscription 2e des Hautes-Alpes Législature XVe et XVIe (Cinquième République) Groupe politique RE Prédécesseur Claire Bouchet 18 juin 2002 – 26 août 2020(18 ans, 2 mois et 8 jours) Élection 16 juin 2002 Réélection 17 juin 200717 juin 201218 juin 2017 Circonscr…

Ираклеониты — ученики гностика Ираклеона (II век). Упоминаются как особая секта Епифанием и Августином; при крещении и миропомазании они соблюдали обряд помазания елеем и при этом произносили воззвания на арамейском языке, которые должны были освободить душу от власти �…