|
Amlexanox
Amlexanox (trade name Aphthasol) is an anti-inflammatory antiallergic immunomodulator used to treat recurrent aphthous ulcers (canker sores), and (in Japan) several inflammatory conditions. This drug has been discontinued in the U.S.[1] Medical usesAmlexanox is the active ingredient in a common topical treatment for recurrent aphthous ulcers of the mouth (canker sores),[2] reducing both healing time[3] and pain.[4] Amlexanox 5% paste is well tolerated,[5] and is typically applied four times per day directly on the ulcers.[3] A 2011 review found it to be the most effective treatment of the eight treatments investigated for recurrent canker sores.[6] It is also used to treat ulcers associated with Behçet disease.[7] In Japan, it is used to treat bronchial asthma, allergic rhinitis and conjunctivitis.[8] ContraindicationsThe drug is contraindicated in those with known allergies to it.[3] Adverse effectsAmlexanox may cause a slightly painful stinging or burning sensation, nausea or diarrhea.[3] Mechanism of actionIts mechanism of action is not well-determined, but it might inhibit inflammation by inhibiting the release of histamine and leukotrienes.[8] It has been shown to selectively inhibit TBK1 and IKK-ε, producing reversible weight loss and improved insulin sensitivity, reduced inflammation and attenuated hepatic steatosis without affecting food intake in obese mice.[9] It produced a statistically significant reduction in glycated hemoglobin and fructosamine in obese patients with type 2 diabetes and nonalcoholic fatty liver disease[10] ChemistryThe chemical itself is an odorless, white to yellowish-white powder.[8] The 5% preparation for patient use is an adherent beige paste,[3][8] and it is also available in some countries as a tablet that adheres to the ulcer in the mouth.[4] PharmacokineticsAmlexanox applied to an aphthous ulcer is largely absorbed through the gastrointestinal tract; an insignificant amount enters the bloodstream through the ulcer itself. After a single 100 mg dose, mean maximum serum concentration occurs 2.4 +/- 0.9 hours after application, with a half-life of elimination (through urine) of 3.5 +/- 1.1 hours. With multiple daily applications (four doses per day), steady state serum levels occur after one week, with no accumulation occurring after four weeks.[8] HistoryThe patent for its use as a treatment for aphthous ulcers was issued in November 1994 to inventors Kakubhai R. Vora, Atul Khandwala and Charles G. Smith, and assigned to Chemex Pharmaceuticals, Inc.[11] Society and cultureEconomicsA 2011 review found a one-week supply of amlexanox 5% paste to cost $30.[6] ResearchA review found that, as of July 2011[update], robust studies investigating its effectiveness alongside other canker sore treatments were still needed.[12] Because it is an inhibitor of the protein kinases TBK1 and IKK-ε,[9] which are implicated in the etiology of type II diabetes and obesity,[13] amlexanox may be a candidate for human clinical trials testing in relation to these diseases.[9] Synthesis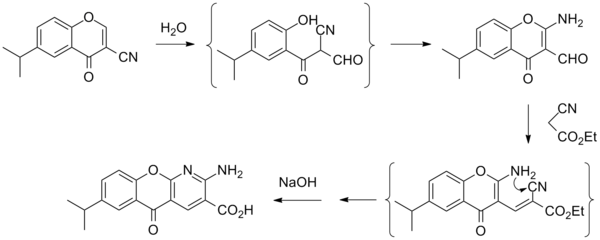 References
Information related to Amlexanox |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
