Dronedarone
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

This article is about the Hong Kong film. For the 2010 American documentary, see Bad Blood: A Cautionary Tale. 2010 Hong Kong filmBad BloodFilm posterTraditional Chinese滅門Simplified Chinese灭门Hanyu PinyinMiè MénJyutpingMit6 Mun4 Directed byDennis S.Y. LawWritten byDennis S.Y. LawProduced byDennis S.Y. LawStarringSimon YamBernice LiuAndy OnLuxia JiangKen LoChan Wai-ManXiong Xin XinPinky CheungLai Lok-YiAmy ChumWong Tin-lamCheung Siu-faiLam SuetCinematographyHerman YauEdited byAzrael…

Geografi Turki BenuaEurasiaKawasanEropa Tenggara dan Asia BaratKoordinat39°00′N 35°00′E / 39.000°N 35.000°E / 39.000; 35.000WilayahPeringkat 37783.562 km² (302.535 mil²)98% daratan 2 % perairanPerbatasanTotal perbatasan darat: 2648 km Armenia 268 km, Azerbaijan 9 km, Bulgaria 240 km, Georgia 252 km, Yunani 206 km, Iran 499 km, Irak 352 km, Suriah 822 kmTitik tertinggiGunung Ağrı (Ararat)5,137 mTi…

Don't Sit Down 'Cause I've Moved Your ChairLagu oleh Arctic Monkeysdari album Suck It and SeeSisi-BBrick by BrickDirilis16 April 2011 (2011-04-16)Format7digital download10GenreStoner rock[1]Durasi3:04LabelDominoPencipta Alex Turner Jamie Cook Matt Helders Nick O'Malley ProduserJames FordSampul alternatifMusic videoDon't Sit Down 'Cause I've Moved Your Chair di YouTube Don't Sit Down 'Cause I've Moved Your Chair adalah sebuah lagu dari grup musik indie rock Inggris Arctic Monkeys. La…

Chronologies Données clés 1775 1776 1777 1778 1779 1780 1781Décennies :1740 1750 1760 1770 1780 1790 1800Siècles :XVIe XVIIe XVIIIe XIXe XXeMillénaires :-Ier Ier IIe IIIe Chronologies géographiques Afrique Afrique du Sud, Algérie, Angola, Bénin, Botswana, Burkina Faso, Burundi, Cameroun, Cap-Vert, République centrafricaine, Comores, République du Congo, République démocratique du Congo, Côte d'Ivoire, Djibouti, Égypte, …

Ordo Saudara-Saudara Santa Perawan Maria dari Gunung KarmelSingkatanOrdo Karmel (O.Carm.)Tanggal pendirianakhir abad ke-12TipeOrdo keagamaan KatolikKantor pusatVia Giovanni Lanza, Roma, ItaliaPrior JenderalRm. Míceál O´Neill, O.CarmSitus webwww.ocarm.orgwww.karmelindonesia.org Ordo Saudara-Saudara Santa Perawan Maria dari Gunung Karmel atau Karmelit (biasa disingkat Ordo Karmel; Latin: Ordo Fratrum Beatissimæ Virginis Mariæ de Monte Carmelocode: la is deprecated ) adalah salah satu ordo kea…

1989 comic book story This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: The Man Who Falls – news · newspapers · books · scholar · JSTOR (February 2008) (Learn how and when to remove this template message) The Man Who FallsCover of Secret Origins of the World's Greatest Super-Heroes (1989), trade paperback edition…

Speed JetCroazia Jet in servizio per SNAV a Spalato nel 2011Descrizione generale Tipocatamarano Ro-Pax ad alta velocità ClasseAutoexpress ProprietàSweFerry (1996-1998)Scandlines (1998-2002)SNAV (2002-2015)Golden Sun Petroleum SA. (2015-2022)SeaJets (2022- ) Porto di registrazione Fremantle (1996) Malmö (1996-2000) Algeciras (2000-2002) Panama (2002-2015) Puerto La Cruz (2015-2022) Limassol (2022- ) Identificazionenominativo intern.le ITU: 5BEY4(Five-Bravo-Echo-Yankee-Four) numero MMSI: 212454…

Fulham FCNama lengkapFulham Football ClubJulukanThe Cottagers, The Whites, The Lilywhites.Berdiri21 Desember 1879; 144 tahun lalu (1879-12-21) (bernama Fulham St Andrew's Church Sunday School)[1]StadionCraven Cottage(Kapasitas: 25.700 (meningkat menjadi 30.000)[2])Pemilik Shahid Khan[3]Ketua Shahid Khan[3]Manajer Marco SilvaLigaLiga Utama Inggris2022-2023Liga Utama Inggris, ke-10 dari 20Situs webSitus web resmi klub Kostum kandang Kostum tandang Kostum k…

العلاقات الوسط أفريقية المالطية جمهورية أفريقيا الوسطى مالطا جمهورية أفريقيا الوسطى مالطا تعديل مصدري - تعديل العلاقات الوسط أفريقية المالطية هي العلاقات الثنائية التي تجمع بين جمهورية أفريقيا الوسطى ومالطا.[1][2][3][4][5] مقارنة بين البلد…

English businessman and politician A c. 1843 portrait of Walmsley Sir Joshua Walmsley (1794–1871) was an English businessman and Liberal Party politician. Early life and education The son of John Walmsley, an architect, builder and marble mason,[1] he was born in Liverpool on 29 September 1794, and educated at Knowsley, Lancashire, and Eden Hall, Westmorland. Career Following the death of his father in 1807, Walmsley became a teacher in Eden Hall School, and after returning to Li…

تلغرافمعلومات عامةصنف فرعي من اتصالات الصناعة بريد تاريخ هذا الموضوع history of telegraphy in Australia (en) يمارسها برقياتي يستعمل برقية تعديل - تعديل مصدري - تعديل ويكي بيانات ملف خارجي «إشارة تلغرافية» من تصدير مكتب القاهرة 1943م البرق[1] (بالإنجليزية: Telegraphy) أو تعريباً: التلغراف[2] …

Lunar robotic craft developed by ISRO Moon Impact Probe being integrated with Chandrayaan-1 orbiter Moon Impact Probe being worked on before integration with orbiter The Moon Impact Probe (MIP) developed by the Indian Space Research Organisation (ISRO), India's national space agency, was a lunar probe that was released by ISRO's Chandrayaan-1 lunar remote sensing orbiter which in turn was launched, on 22 October 2008, aboard a modified version of ISRO's Polar Satellite Launch Vehicle. It discove…
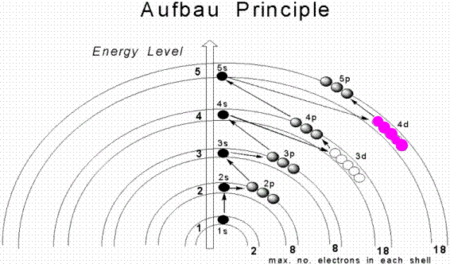
Représentation plus courante du diagramme : ici chaque flèche rouge diagonale correspond à une valeur de n + ℓ. Disposition en couches selon le principe d'Aufbau. La règle de Klechkowski, du nom du chimiste russe Vsevolod Kletchkovski, également appelée règle de Madelung[1] (notamment dans les pays anglo-saxons), du nom du physicien allemand Erwin Madelung, est une méthode empirique permettant de prédire avec une assez bonne précision l'ordre de remplissage des électrons dans l…
Ben 10: OmniverseBerkas:Ben10OmiverseBoxart.png PublikasiNA: 13 November 2012EU: 30 November 2012AU: 30 November 2012Wii UNA: 18 November 2012EU: 30 November 2012AU: 30 November 2012GenreAksi bertarungLatar tempatBen 10 universe (en) Karakteristik teknisPlatformNintendo DS, Wii, PlayStation 3 dan Xbox 360 MesinQuantum3Modepermainan video multipemain dan Permainan video pemain tunggal FormatCakram Blu-ray, Cakram optik Nintendo, Nintendo game card (en), DVD dan unduhan digital Format kode Daftar …

Marrying My Daughter TwicePoster promosiGenreKomediDramaKeluargaDitulis olehAhn Seo-jungSutradaraAhn Kil-hoPemeranYang Jin-sung Seo Ha-joon Park Soon-chunNegara asalKorea SelatanBahasa asliKoreaJmlh. episode120Rilis asliJaringanSBSRilis04 Januari 2016 (2016-01-04) Marrying My Daughter Twice[1] (Hangul: 내 사위의 여자; lit. My Son-in-Law's Woman) adalah serial televisi Korea Selatan tahun 2016 yang dibintangi oleh Yang Jin-sung, Seo Ha-joon, dan Park Soon-chun…

Coupe d'Asie des nations 1956 Généralités Sport Football Organisateur(s) AFC Édition 1re Lieu(x) Hong Kong Date du 1er septembre 1956au 15 septembre 1956 Participants 4 Palmarès Vainqueur Corée du Sud (1) Deuxième Israël Troisième Hong Kong Buts 27 (4,5/match) Meilleur(s) buteur(s) Nahum Stelmach (4) Navigation Corée du Sud 1960 modifier La Coupe d'Asie des nations 1956 a eu lieu à Hong Kong en septembre 1956 et fut remportée par la Corée du Sud. Tournoi de qualification Article dé…

Chemical compound Nipecotic acidIdentifiers IUPAC name Piperidine-3-carboxylic acid CAS Number498-95-3PubChem CID4498IUPHAR/BPS4564ChemSpider4342UNII1U1QTN40SYChEMBLChEMBL277498ECHA InfoCard100.007.159 Chemical and physical dataFormulaC6H11NO2Molar mass129.159 g·mol−13D model (JSmol)Interactive image SMILES C1CC(CNC1)C(=O)O Nipecotic acid is a GABA uptake inhibitor[1] used in scientific research.[2][3] See also Deramciclane Tiagabine Niacin References ^ Macdonald,…

American sprinter Harry Gissing, wearing the Winged Foot of the New York Athletic Club. Harry Gissing, wearing the Winged Fist of the Irish American Athletic Club, 1911. Harry E. Gissing (December 3, 1890 - November 29, 1963) was an American track and field athlete, a member of the New York Athletic Club, Mohawk Athletic Club, and the Irish American Athletic Club. In 1911, he was part of a world's record setting team in the 4x400 meter relay race. Biography In 1908, Gissing won the A.A.U half-mi…

Minnesota Golden Gophers men's gymnasticsFounded1938UniversityUniversity of MinnesotaHead coachMike BurnsConferenceBig Ten ConferenceHome arenaMaturi Pavilion (Capacity: 5,700)ColorsMaroon and gold[1] NCAA Tournament appearances1938, 1939, 1940, 1941, 1942, 1948, 1949, 1950, 1951, 1952, 1953, 1954, 1955, 1956, 1957, 1959, 1960, 1963, 1964, 1976, 1977, 1978, 1979, 1982, 1984, 1987, 1988, 1989, 1990, 1991, 1992, 1993, 2001, 2002, 2003, 2004, 2005, 2006, 2007…

Telephone numbers in SwedenLocation of Sweden in dark greenLocationCountrySwedenContinentEuropeRegulatorSwedish Post and Telecom AuthorityTypeOpenNSN length7 to 13 digitsNumbering planSweden National Numbering PlanLast updated22 May 2018Access codesCountry code+46International access00Long-distance0 In Sweden, the area codes are, including the leading 0, two, three or four digits long, with larger towns and cities having shorter area codes permitting a larger number of telephone numbers in the e…
