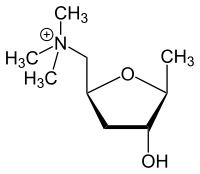
Muscarine
| ||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

LestiLesti pada tahun 2021LahirLestiani[1]5 Agustus 1999 (umur 24)Cianjur, Jawa Barat, IndonesiaKebangsaanIndonesiaNama lainLesti KejoraLesti DALestiAlmamaterUniversitas Mercu BuanaPekerjaanPenyanyiAktrisPresenterPengusahaTahun aktif2014–sekarangKota asalCianjur, Jawa BaratSuami/istriRizky Billar (m. 2021)Anak1Karier musikGenreDangdutMelayuSundaPopInstrumenVokalTahun aktif2014—sekarangLabelTrinity Optima3D Entertainment Lestiani, ata…

Peta infrastruktur dan tata guna lahan di Komune Lamarche. = Kawasan perkotaan = Lahan subur = Padang rumput = Lahan pertanaman campuran = Hutan = Vegetasi perdu = Lahan basah = Anak sungaiLamarche merupakan sebuah komune di departemen Vosges yang terletak pada sebelah timur laut Prancis. Lihat pula Komune di departemen Vosges Referensi INSEE lbsKomune di departemen Vosges Les Ableuvenettes Ahéville Aingeville Ainvelle Allarmont Ambacourt Ameuvell…

1969 novel by Jackie Collins This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: The Stud novel – news · newspapers · books · scholar · JSTOR (April 2008) (Learn how and when to remove this template message) The Stud First imageAuthorJackie CollinsCountryUnited KingdomLanguageEnglishGenreFictionPublished1969Pu…

Sprinter Jamaika bernama Usain Bolt [1] Determinisme nominatif adalah sebuah hipotesis yang menyatakan bahwa individu cenderung tertarik pada bidang pekerjaan yang sesuai dengan nama individu tersebut. Istilah ini pertama kali muncul di majalah New Scientist tahun 1994, ketika kolom Feedback membahas sejumlah studi tentang kecocokan bidang pekerjaan dengan nama keluarga seseorang. Contohnya meliputi buku karangan Daniel Snowman yang membahas penjelajahan kutub dan sebuah artikel tentang …

Rebels on the RunAlbum studio karya Montgomery GentryDirilis18 Oktober 2011 (2011-10-18)[1][2]GenreCountryDurasi36:41LabelAverage JoesProduserMichael Knox[3]Kronologi Montgomery Gentry For Our Heroes(2009) Rebels on the Run(2011) Playlist: The Very Best of Montgomery GentryString Module Error: Match not foundString Module Error: Match not found Singel dalam album Rebels on the Run Where I Come FromDirilis: 25 Juli 2011 (2011-07-25) So Called LifeDirilis: 30 …

This article is a list of notable encyclopedic persons, students, alumni, faculty, and academic affiliates associated with Santa Clara University in Santa Clara, California United States. University presidents Rev. Aloysius Varsi, SJ president 1868–76 Rev. John Pinasco, SJ president 1880–83 & 88–93 John Nobili, S.J., 1851–56 Nicholas Congiato, S.J., 1856–57 Felix Cicaterri, S.J., 1857–61 Burchard Villiger, S.J., 1861–65 Aloysius Masnata, S.J., 1865–68 Aloysius Varsi, S.J., 18…

本條目存在以下問題,請協助改善本條目或在討論頁針對議題發表看法。 此條目需要补充更多来源。 (2018年3月17日)请协助補充多方面可靠来源以改善这篇条目,无法查证的内容可能會因為异议提出而被移除。致使用者:请搜索一下条目的标题(来源搜索:羅生門 (電影) — 网页、新闻、书籍、学术、图像),以检查网络上是否存在该主题的更多可靠来源(判定指引)。 此�…

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Maret 2020. Berikut ini adalah daftar wali kota Bonn yang terletak di Nordrhein-Westfalen, Jerman. Daftar ini termasuk wali kota (Oberbürgermeister) sejak tahun 1800 dan juga pemimpin kota (Oberstadtdirektoren) dari tahun 1947 sampai 1996 ketika jabatan ini dihapuskan…

Radio station in Fair Oaks–Sacramento, California KKDOFair Oaks, CaliforniaUnited StatesBroadcast areaSacramento metro areaFrequency94.7 MHz (HD Radio)BrandingAlt 94-7ProgrammingLanguage(s)EnglishFormatAlternative rockSubchannelsHD2: Channel QOwnershipOwnerAudacy, Inc.(Audacy License, LLC, as Debtor-in-Possession)Sister stationsKIFMKRXQKSEGKSFMKUDLHistoryFirst air dateNovember 25, 1970 (1970-11-25)Former call signsKNIS (1970–90)KRWR (1990–92)KIZS (1992–94)KTHX (1994–97)K…

Viktor Tsyhankov Informasi pribadiNama lengkap Viktor Vitaliyovych TsyhankovTanggal lahir 15 November 1997 (umur 26)Tempat lahir Nahariya, IsraelTinggi 178 m (584 ft 0 in)[1]Posisi bermain MidfielderInformasi klubKlub saat ini Dynamo KyivNomor 15Karier junior2009–2010 Nyva Vinnytsia2011–2016 Dynamo KyivKarier senior*Tahun Tim Tampil (Gol)2016– Dynamo Kyiv 116 (54)Tim nasional‡2012 Ukraine U16 4 (2)2012–2014 Ukraine U17 20 (8)2014–2016 Ukraine U19 10 (1)201…

Французские монеты евро — современные денежные знаки Франции. Всего существует три дизайна национальной стороны монеты: на монетах мелкого номинала изображён портрет Марианны, символа Французской республики, на монетах покрупнее изображена сеятельница, ранее распол…

Fox News ChannelCaractéristiquesCréation 7 octobre 1996Propriétaire Fox CorporationFormat d'image 480i, 720pLangue AnglaisPays États-UnisStatut Information en continuSiège social New YorkSite web foxnews.comDiffusionAire Diffusion Satellite, câble, IPTVmodifier - modifier le code - modifier Wikidata Fox News Channel (prononcé en anglais : /fɑks nuːz ˈt͡ʃænəl/)[1], souvent appelée Fox News, est une chaîne de télévision d'information en continu américaine, lancée le 7 octo…

La Hulpe La rivière Argentine devant le parc du château Solvay (au fond) à La Hulpe. Héraldique Drapeau Administration Pays Belgique Région Région wallonne Communauté Communauté française Province Province du Brabant wallon Arrondissement Nivelles Bourgmestre Christophe Dister (MR)(LdB) Majorité Liste du Bourgmestre SiègesECOLODéFIListe du BourgmestreListe des Citoyens 1931132 Section Code postal La Hulpe 1310 Code INS 25050 Zone téléphonique 02 Démographie Genti…

Растительный мир Украины — один из важнейших компонентов природы, который представлен совокупностью различных растительных сообществ, произрастающих на территории Украины. Большое разнообразие климатических условий и почвенного покрова, а также влияния прошлых гео…

Erovnuli Liga 2017 Competizione Erovnuli Liga Sport Calcio Edizione 29ª Organizzatore GFF Date dal 4 marzo 2017al 26 novembre 2017 Luogo Georgia Partecipanti 10 Formula Girone all'italiana Risultati Vincitore T'orp'edo Kutaisi(4º titolo) Secondo Dinamo Tbilisi Retrocessioni Dinamo BatumiShukura Kobuleti Statistiche Miglior marcatore Irakli Sikharulidze (25) Incontri disputati 180 Gol segnati 506 (2,81 per incontro) Cronologia della competizione 2016 2018 Manuale Chikhur…

Battle between British and Ottoman forces in 1917 31°29′21″N 34°28′25″E / 31.4893°N 34.4737°E / 31.4893; 34.4737 Third Battle of GazaPart of the Middle Eastern theatre of World War IDate1–2 November 1917(1 day)LocationGaza, southern PalestineResult Allied victory[1] Ottoman garrison abandons Gaza on 7 November as a result of fighting during the Battle of Tel el Khuweilfe and the Battle of Hareira and SheriaBelligerents British Empire …

Ini adalah nama Melayu; nama Sabu merupakan patronimik, bukan nama keluarga, dan tokoh ini dipanggil menggunakan nama depannya, Mohamad. Kata bin (b.) atau binti (bt.), jika digunakan, berarti putra dari atau putri dari. Yang Berhormat Datuk Seri HajiMohamad SabuSMW APمحمد سابو Menteri Pertanian dan Ketahanan Pangan MalaysiaPetahanaMulai menjabat 3 Desember 2022Perdana MenteriAnwar IbrahimPendahuluRonald KiandeePenggantiPetahanaMenteri Pertahanan MalaysiaMasa jabatan21 Mei 2018 …

Basilica di San RemigioLa Basilica nel 2020Stato Francia RegioneChampagne-Ardenne LocalitàReims Coordinate49°14′35″N 4°02′31″E / 49.243056°N 4.041944°E49.243056; 4.041944Coordinate: 49°14′35″N 4°02′31″E / 49.243056°N 4.041944°E49.243056; 4.041944 ReligioneCattolica TitolareSan Remigio Arcidiocesi Reims Stile architettonico Romanico Gotico Inizio costruzioneXI secolo CompletamentoXIII secolo Modifica dati su Wikidata · Manuale La…

2013 single by Beyoncé BlowSong by Beyoncéfrom the album Beyoncé ReleasedDecember 13, 2013Studio Jungle City Studios Oven Studios Genre Disco funk R&B Length5:09Composer(s) Pharrell Williams Beyoncé Knowles Timothy Mosley Jerome Harmon Lyricist(s) Williams Knowles James Fauntleroy Terius Nash Justin Timberlake Producer(s) Beyoncé Pharrell Williams Timbaland J-Roc Blow is a song recorded by American singer Beyoncé from her self-titled fifth studio album (2013). It was written by Beyonc�…

Peta menunjukan lokasi MacArthur MacArthur adalah munisipalitas yang terletak di provinsi Leyte, Filipina. Pada tahun 2010, munisipalitas ini memiliki populasi sebesar 17.608 jiwa atau 3.681 rumah tangga. Pembagian wilayah Secara administratif MacArthur terbagi atas 31 barangay, yaitu: Batug Burabod Capudlosan Casuntingan Causwagan Danao General Luna Kiling Lanawan Liwayway Maya Oguisan Osmeña Palale 1 Palale 2 Poblacion District 1 Poblacion District 2 Poblacion District 3 Pongon Quezon Romuald…