Oxide
|
Read other articles:

Marsha Milan LondohLahirMarsha Milan Londoh[1]6 Desember 1985 (umur 38)Michigan USATamparuli, Sabah, MalaysiaKebangsaan MalaysiaPekerjaanpenyanyi, pembawa acara, artis, pengisi suaraTahun aktif2005 - sekarangSuami/istriMohamed Shaiful IsmailKerabatNorlinda Nanuwil Marsha Milan Londoh adalah seorang penyanyi dan aktris berkebangsaan Malaysia yang dikenal melalui sebuah acara realitas pencarian bakat Akademi Fantasia musim ketiga. Marsha merupakan penyanyi yang d…

Youssef En-Nesyri En-Nesyri bermain untuk Leganés pada 2018Informasi pribadiNama lengkap Youssef En-Nesyri[1]Tanggal lahir 1 Juni 1997 (umur 26)[1]Tempat lahir Fez, MarokoTinggi 188 cm (6 ft 2 in)[2]Posisi bermain PenyerangInformasi klubKlub saat ini SevillaNomor 15Karier junior2010–2011 Maghreb de Fès2011–2015 Mohammed VI Football AcademyKarier senior*Tahun Tim Tampil (Gol)2016–2017 Málaga B 29 (16)2016–2018 Málaga 38 (5)2018–2020 Legan�…

العلاقات البليزية التنزانية بليز تنزانيا بليز تنزانيا تعديل مصدري - تعديل العلاقات البليزية التنزانية هي العلاقات الثنائية التي تجمع بين بليز وتنزانيا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: وجه المقارنة بليز تن…

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Ahdi Muqsith – berita · surat kabar · buku · cendekiawan · JSTOR Artikel biografi ini ditulis menyerupai resume atau daftar riwayat hidup (Curriculum Vitae). Tolong bantu perbaiki agar netral dan ensikloped…
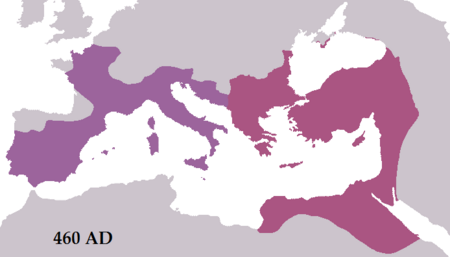
Leo IKaisar Kekaisaran Romawi TimurPotret kerajaan Leo I di Museum LouvreBerkuasa457–474Penobatan7 Februari 457PendahuluMarcianPenerusLeo IIKelahiran401Kematian18 Januari 474 (usia 73)Nama lengkapFlavius Valerius Leo AugustusIstriVerinaAnakAriadne, Leontia, putra yg tak bernama Leo I (bahasa Latin: Flavius Valerius Leo Augustus) (401 – 18 Januari 474) merupakan seorang Kaisar Romawi Timur dari tahun 457 sampai dengan tahun 474. Seorang penduduk asli Dacia Aureliana didekat Trakia yang be…

1909 Queensland state election ← 1908 2 October 1909 1912 → All 72 seats in the Legislative Assembly of Queensland37 Assembly seats were needed for a majorityTurnout72.67 ( 6.24 pp) First party Second party Leader William Kidston David Bowman Party Liberal Labour Leader's seat Rockhampton Fortitude Valley Last election New party 23 seats, 29.80% Seats won 41 27 Seat change 4 4 Popular vote 107,370 77,712 Percentage 50.91% 36.58% Swing …

Taman Nasional Gunung MerbabuGunung Merbabu dari arah Selo, BoyolaliTN Gunung MerbabuLokasi di JawaLetakJawa Tengah, IndonesiaKota terdekatKota BoyolaliKota MagelangKota SalatigaKoordinat7°30′0″S 110°24′0″E / 7.50000°S 110.40000°E / -7.50000; 110.40000Koordinat: 7°30′0″S 110°24′0″E / 7.50000°S 110.40000°E / -7.50000; 110.40000Luas5.725 HaDidirikan2004Pihak pengelolaBalai TN Gunung Merbabu Taman Nasional Gunung Merbabu (TNGMb…

This biography of a living person needs additional citations for verification. Please help by adding reliable sources. Contentious material about living persons that is unsourced or poorly sourced must be removed immediately from the article and its talk page, especially if potentially libelous.Find sources: Fernando Mon – news · newspapers · books · scholar · JSTOR (March 2010) (Learn how and when to remove this template message) Fernando MonBackground i…

District in Punjab, India District of Punjab in IndiaJalandhar districtDistrict of PunjabSerai NurmahalLocation in PunjabCountry IndiaStatePunjabRegionDoabaNamed forArea inside the waterHeadquartersJalandharGovernment • Administrator of DistrictSh. Ghanshyam ThoriArea • Total2,632 km2 (1,016 sq mi)Population (2017)[‡] • Total4,193,590 • Density1,600/km2 (4,100/sq mi)Languages • OfficialPunjabiTime…

Daftar keuskupan di Lituania adalah sebuah daftar yang memuat dan menjabarkan pembagian terhadap wilayah administratif Gereja Katolik Roma yang dipimpin oleh seorang uskup ataupun ordinaris di Lituania . Konferensi uskup di wilayah Lituania bergabung dalam Konferensi Waligereja Lituania. Saat ini terdapat 8 buah yurisdiksi, di mana 2 merupakan keuskupan agung, 5 merupakan keuskupan sufragan, dan 1 merupakan ordinariat militer. Daftar keuskupan Provinsi Gerejawi Kaunas Keuskupan Agung Kaunas: Lio…

この項目には、一部のコンピュータや閲覧ソフトで表示できない文字が含まれています(詳細)。 数字の大字(だいじ)は、漢数字の一種。通常用いる単純な字形の漢数字(小字)の代わりに同じ音の別の漢字を用いるものである。 概要 壱万円日本銀行券(「壱」が大字) 弐千円日本銀行券(「弐」が大字) 漢数字には「一」「二」「三」と続く小字と、「壱」「弐」…

ACE inhibitor medication RamiprilClinical dataTrade namesAltace, othersAHFS/Drugs.comMonographMedlinePlusa692027License data US DailyMed: Ramipril Routes ofadministrationBy mouthATC codeC09AA05 (WHO) Legal statusLegal status CA: ℞-only UK: POM (Prescription only) US: ℞-only Pharmacokinetic dataBioavailability28%Protein binding73% (ramipril)56% (ramiprilat)MetabolismLiver, to ramiprilatElimination half-life13 to 17 hoursExcretionKidney (60%) and fecal (40%)…
2020年夏季奥林匹克运动会波兰代表團波兰国旗IOC編碼POLNOC波蘭奧林匹克委員會網站olimpijski.pl(英文)(波兰文)2020年夏季奥林匹克运动会(東京)2021年7月23日至8月8日(受2019冠状病毒病疫情影响推迟,但仍保留原定名称)運動員206參賽項目24个大项旗手开幕式:帕维尔·科热尼奥夫斯基(游泳)和马娅·沃什乔夫斯卡(自行车)[1]闭幕式:卡罗利娜·纳亚(皮划艇)[2…

莎拉·阿什頓-西里洛2023年8月,阿什頓-西里洛穿著軍服出生 (1977-07-09) 1977年7月9日(46歲) 美國佛羅里達州国籍 美國别名莎拉·阿什頓(Sarah Ashton)莎拉·西里洛(Sarah Cirillo)金髮女郎(Blonde)职业記者、活動家、政治活動家和候選人、軍醫活跃时期2020年—雇主內華達州共和黨候選人(2020年)《Political.tips》(2020年—)《LGBTQ國度》(2022年3月—2022年10月)烏克蘭媒體�…

1992 greatest hits album by Curtis Mayfield and The ImpressionsThe Anthology 1961-1977Greatest hits album by Curtis Mayfield and The ImpressionsReleasedDecember 8, 1992Recorded1961-1977GenreSoul, funk[1]Length132:47LabelCurtomProducerCurtis MayfieldAndy McKaieJohnny Pate The Anthology 1961-1977 is a compilation album of songs by Curtis Mayfield when he was with the Impressions and when he was solo. Of the 40 tracks, 30 are from Mayfield's time with the Impressions. The album incl…

Iman Budhi SantosaSantosa pada saat peluncuran bukunya Sesanti Tedhak Siti, 2015Lahir(1948-03-28)28 Maret 1948Kauman, Magetan, Jawa Timur, IndonesiaMeninggal10 Desember 2020(2020-12-10) (umur 72)Kota Yogyakarta, Yogyakarta, IndonesiaKebangsaanIndonesiaAlmamaterAkademi Pertanian, SemarangPekerjaanPenyairSuami/istriSri Maryati (1971–1978)Anak4 Iman Budhi Santosa (28 Maret 1948 – 10 Desember 2020), yang umumnya dikenal sebagai IBS, adalah seorang penulis Indonesia yang berbas…

Ввод пароля в Википедии с помощью экранной лупы Экранная лупа — это компьютерная программа, которая взаимодействует с графическим выводом компьютера для увеличения части изображения на экране. Экранная лупа может использоваться слабовидящими и является одним из вид�…

معدات وقاية شخصية تشمل جهاز تنفس شخصي مضغوط من أجل الاستخدام في حالات الخطورة فورية للحياة أو الصحة. خطورة فورية للحياة أو الصحة (يرمز له IDLH من immediately dangerous to life or health) هو مصطلح معرف من المعهد الوطني للسلامة والصحة المهنية (NIOSH) على أنه التعرّض لملوثات وشوائب محمولة في الهواء وا�…

Indian news agency The Press Trust of India Ltd.PTI Head Office on Parliament Street, New Delhi.Company typeNon-profit cooperative[1]IndustryNews mediaFounded27 August 1947; 76 years ago (1947-08-27)HeadquartersPTI Building, 4, Parliament Street, New Delhi, India[2]Area servedWorldwideKey peopleAveek Sarkar(Chairman)Vijay Joshi(Editor-in-Chief)Revenue ₹1.73 billion (US$21 million)[3] (2016–17)Number of employees1,000+ (2014)DivisionsPT…

Suburb of Kalgoorlie, Western AustraliaBoulderKalgoorlie, Western AustraliaBoulder Town HallCoordinates30°46′55″S 121°29′19″E / 30.78194°S 121.48861°E / -30.78194; 121.48861Population4,872 (SAL 2021)[1]Postcode(s)6432Elevation355 m (1,165 ft)Area4.5 km2 (1.7 sq mi)Location 4.2 km (3 mi) S of Kalgoorlie 597 km (371 mi) E of Perth 386 km (240 mi) N of Esperance LGA(s)City of Kalgoorlie–Boulder…




















