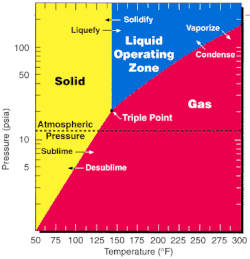
Uranium hexafluoride
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

The Warren is a 13.5-hectare (33-acre) nature reserve in St Mary Cray in the London Borough of Bromley. It is a Site of Borough Importance for Nature Conservation, Grade I, and is managed by the London Wildlife Trust.[1][2] The site is mainly ancient oak and silver birch woodland with a ground flora of bracken, foxgloves and bluebells. The wood is open, with grass clearings, and there is a pond which has rare London plants such as blue fleabane and hare's-foot clover. Birds inclu…

Tom Bethea Nazionalità Stati Uniti Pugilato Categoria Pesi medi e mediomassimi Termine carriera 1978 Carriera Incontri disputati Totali 46 Vinti (KO) 22 (8) Persi (KO) 21 (12) Pareggiati 3 Modifica dati su Wikidata · Manuale Tom Bethea, detto The Bomb (New York, 24 gennaio 1944), è un ex pugile statunitense, sfidante al titolo mondiale dei pesi medi e due volte avversario di Nino Benvenuti. Carriera Venticinquenne, nel maggio 1969 il newyorchese è ingaggiato da Nino Benvenu…

Основная статья: Спорт в России Хоккей с шайбой – один из самых популярных видов спорта в России. В стране существуют две взрослые профессиональные хоккейные лиги и две молодёжные лиги. Развит детско-юношеский, женский и студенческий хоккей. Федерация хоккея России (ФХР) о…

Liga Eropa UEFA 2012–13Stadion Amsterdam Arena yang menyelenggarakan pertandingan final.Informasi turnamenJadwalpenyelenggaraan20 September 2012 – 15 Mei 2013 (babak utama)5 Juli – 30 Agustus 2012 (babak kualifikasi)Jumlahtim peserta48+8 (babak utama)161+32 (total) (dari 53 asosiasi)Hasil turnamenJuara Chelsea (gelar ke-1)Tempat kedua BenficaStatistik turnamenJumlahpertandingan205Jumlah gol521 (2,54 per pertandingan)Jumlahpenonton4.174.756 (20.365 per pertandingan)Pencetak g…

Mathys Tel Informasi pribadiTanggal lahir 27 April 2005 (umur 18)Tempat lahir Sarcelles, PrancisTinggi 183 cm (6 ft 0 in)[1]Posisi bermain PenyerangInformasi klubKlub saat ini Bayern MünchenNomor 39Karier junior2012–2016 JS Villiers-le-Bel2016–2017 Paris FC2017–2019 AS Jeunesse Aubervilliers2019–2020 Montrouge FC 922020–2022 RennesKarier senior*Tahun Tim Tampil (Gol)2021–2022 Rennes B 6 (6)2021–2022 Rennes 7 (0)2022– Bayern München 28 (8)Tim nasional…

Mehdi Mostefa Informasi pribadiNama lengkap Mehdi Mostefa SbaaTanggal lahir 30 Agustus 1983 (umur 40)Tempat lahir Dijon, PrancisTinggi 1,81 m (5 ft 11+1⁄2 in)Posisi bermain GelandangInformasi klubKlub saat ini AC AjaccioNomor 14Karier junior1994–1997 Fontaine-les-Dijon FC1997–1998 Dijon1998–2003 MonacoKarier senior*Tahun Tim Tampil (Gol)2004–2005 Valence 37 (1)2005–2006 Montluçon - (-)2006–2007 Sète 31 (4)2007–2011 Nîmes 140 (7)2011– Ajaccio 36 (3)Tim…

Halaman ini berisi artikel tentang unsur kimia. Untuk kegunaan lain, lihat Neon (disambiguasi). 10NeNeonGas neon dalam tabung lucutan, yang disebut lampu neon. Garis spektrum neonSifat umumNama, lambangneon, NePengucapan/nèon/[1] Penampilangas tak berwarna, akan menjadi merah-jingga jika diletakkan pada medan listrik bertegangan tinggiNeon dalam tabel periodik 10Ne Hidrogen Helium Lithium Berilium Boron Karbon Nitrogen Oksigen Fluor Neon Natrium Magnesium Aluminium Silikon Fosfor S…

Artikel ini bukan mengenai [[:Kapal perusak kelas Akatsuki dari masa Perang Rusia-Jepang]]. Untuk kapal lain dengan nama serupa, lihat Kapal perusak kelas Akatsuki. Akatsuki di perairan China, tahun 1938 Tentang kelas Nama:Kelas AkatsukiPembangun:*Sasebo Naval Arsenal Maizuru Naval Arsenal Uraga Dock Company Fujinagata ShipyardsOperator: Angkatan Laut Kekaisaran Jepang Angkatan Laut Uni SovietDidahului oleh:Kapal perusak kelas-FubukiDigantikan oleh:Kapal perusak kelas-HatsuharuBertugas…

Lambang Kabupaten Pulau Taliabu, Provinsi Maluku Utara. Berikut ini adalah daftar kecamatan dan desa di Kabupaten Pulau Taliabu, Provinsi Maluku Utara, Indonesia. Kabupaten Pulau Taliabu terdiri atas 8 kecamatan dan 71 desa dengan luas wilayah 1.469,93 km² dan jumlah penduduk 56.202 jiwa (2017). Kode Wilayah Kabupaten Pulau Taliabu adalah 82.08.[1][2][3][4][5] Kode Wilayah Nama Kecamatan Ibu kota Jumlah Desa Daftar Desa 82.08.01 Taliabu Barat Bobong …

Honolulu, the capital of Hawaii, is a U.S. city. As of late 2020, Honolulu had 92 high-rise buildings over 300 feet (91 meters) in height, with four more under construction.[1] The first high-rise that exceeded 350 ft was the Ala Moana Hotel built in 1970. The next high-rise was the Yacht Harbor Towers followed by the Hawaii Monarch Hotel and the Discovery Bay Center. This was the beginning of the construction boom in the city. At the same time business and finance also boomed. Duri…

artikel ini tidak memiliki pranala ke artikel lain. Tidak ada alasan yang diberikan. Bantu kami untuk mengembangkannya dengan memberikan pranala ke artikel lain secukupnya. (Pelajari cara dan kapan saatnya untuk menghapus pesan templat ini) Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Januari 2023. Berkas:101912 …

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Maret 2024. Gereja Santa Perawan Maria Bunda Kristus Wedi merupakan salah satu gereja yang masuk dalam paroki di bawah Keuskupan Agung Semarang.[1] Gereja ini sering disingkat menjadi Gereja SPM Bunda Kristus Wedi. Gereja SPM Bunda Kristus Wedi. Sumber: Komsos …

هذه المقالة تحتاج للمزيد من الوصلات للمقالات الأخرى للمساعدة في ترابط مقالات الموسوعة. فضلًا ساعد في تحسين هذه المقالة بإضافة وصلات إلى المقالات المتعلقة بها الموجودة في النص الحالي. (مارس 2018) لمعانٍ أخرى، طالع مقاطعة دالاس (توضيح). مقاطعة دالاس الإحداثيات 37°40�…

Golf tour season 2018–19 PGA Tour seasonDurationOctober 4, 2018 (2018-10-04) – August 25, 2019 (2019-08-25)Number of official events46Most wins Brooks Koepka (3) Rory McIlroy (3)FedEx Cup Rory McIlroyMoney list Brooks KoepkaPGA Tour Player of the Year Rory McIlroyPGA Player of the Year Brooks KoepkaRookie of the Year Im Sung-jae← 2017–18 2019–20 → The 2018–19 PGA Tour was the 104th season of the PGA Tour, the main professional golf tour in the United S…

العلاقات الجنوب أفريقية الماليزية جنوب أفريقيا ماليزيا جنوب أفريقيا ماليزيا تعديل مصدري - تعديل العلاقات الجنوب أفريقية الماليزية هي العلاقات الثنائية التي تجمع بين جنوب أفريقيا وماليزيا.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة و�…

PT Zurich Topas LifeJenisJasa keuanganDidirikanJakarta, Indonesia (1986)Kantorpusat Jakarta, IndonesiaTokohkunciChristopher Franz Bendl Presiden DirekturPemilikZurich Insurance Group Mayapada GroupSitus webwww.zurich.co.id Zurich Topas Life atau biasa disebut dengan Zurich Life Indonesia atau Zurich Life adalah perusahaan asuransi jiwa yang berdiri sejak 1986 dan berkantor pusat di Jakarta. Perusahaan sebelumnya bernama Century Lifindo Perdana sejak berdiri hingga tahun 2009. Kemu…
Kim Young-kwangKim Young-kwang in 2014Lahir11 Januari 1987 (umur 37)Incheon, Korea SelatanPendidikanUniversitas Hanyang - Teater dan FilmPekerjaanPemeran, modelTahun aktif2006–sekarangAgenWide-s companyNama KoreaHangul김영광 Hanja金英光 Alih AksaraGim Yeong-gwangMcCune–ReischauerKim Yŏnggwang Kim Young-Kwang (Korea: 김영광code: ko is deprecated ) (lahir 11 Januari 1987) adalah aktor dan model asal Korea Selatan yang membintangi seri televisi KBS, Love Rain[1][…

Untuk versi filmnya, lihat The Lord of the Rings: The Return of the King (film). Cincin kekuasaan Kembalinya Sang Raja (judul bahasa Inggris: The Return of The King) adalah jilid ketiga dan terakhir dari novel epik The Lord of the Rings karya penulis Inggris, J. R. R. Tolkien. Buku ini dibagi menjadi dua bagian (The War of The Ring dan The End of the Third Age) dan pertama kali dirilis di Britania Raya pada 20 Oktober 1955. Dua jilid sebelumnya masing-masing adalah Sembilan Pembawa Cincin dan Du…

Relai atau sambung-lepas adalah suatu peranti yang menggunakan elektromagnet untuk mengoperasikan seperangkat kontak sakelar. Susunan paling sederhana terdiri dari kumparan kawat penghantar yang dililit pada inti besi. Bila kumparan ini diberi tenaga, medan magnet yang terbentuk menarik armatur berporos yang digunakan sebagai pengungkit mekanisme sakelar magnet. Selain menggunakan elektromagnet, relai telah dikembangkan sebagai relai solid state dan relai numerik. Pengaturan pada relai solid sta…

Stasiun Nagayama長山駅Stasiun Nagayama pada April 2018LokasiNishisuijinbira Kaminagayamacho, Toyokawa-shi, Aichi-ken 441-1202JepangKoordinat34°51′53″N 137°25′51″E / 34.8646°N 137.4308°E / 34.8646; 137.4308Koordinat: 34°51′53″N 137°25′51″E / 34.8646°N 137.4308°E / 34.8646; 137.4308Operator JR CentralJalur Jalur IidaLetak14.4 kilometer dari ToyohashiJumlah peron2 peron sampingInformasi lainStatusTanpa stafSejarahDibuka15 Ju…
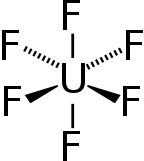

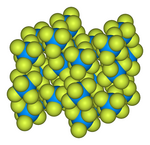





![Ball-and-stick model of the unit cell of uranium hexafluoride[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c6/Uranium-hexafluoride-unit-cell-3D-balls.png/180px-Uranium-hexafluoride-unit-cell-3D-balls.png)
![Bond lengths and angles of gaseous uranium hexafluoride[8]](http://upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Uranium_hexafluoride_dimensions.svg/180px-Uranium_hexafluoride_dimensions.svg.png)