Yield (chemistry)
|
Read other articles:

NASA astronaut image of Aldabra Atoll (Seychelles) in the Indian Ocean Outer Islands (Kepulauan Luar) atau Coralline Seychelles adalah istilah kolektif bagi pulau di Seychelles yang tidak termasuk dalam Tepi Seychelles (Dataran Tinggi Seychelles) yang mendefinisikan lokasi Inner Islands (Kepulauan Dalam). Kepulauan ini berada pada jarak 230–1150 km dari pulau utama Seychelles, Mahé, dan semua formasi karang. Nama lokal untuk kepulauan ini adalah Zil Elwannyen Sesel. Pulau-pulau ini di lu…

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Daftar pesantren di Kabupaten Jombang – berita · surat kabar · buku · cendekiawan · JSTOR Berikut adalah daftar nama pondok pesantren yang ada di Jombang No. Nama Ponpes Alamat 1 Pondok Pesantren Tebuireng …

Artikel ini tidak memiliki referensi atau sumber tepercaya sehingga isinya tidak bisa dipastikan. Tolong bantu perbaiki artikel ini dengan menambahkan referensi yang layak. Tulisan tanpa sumber dapat dipertanyakan dan dihapus sewaktu-waktu.Cari sumber: Akademi Keperawatan Panti Kosala – berita · surat kabar · buku · cendekiawan · JSTOR Akademi Keperawatan Panti KosalaNama lainAKPER Panti KosalaJenisPerguruan Tinggi SwastaDidirikan31 Mei 1991DirekturDra. E…

جزء من سلسلة مقالات سياسة فلسطينفلسطين الدستور القانون الأساسي الفلسطيني (الدستور) الميثاق الوطني حقوق الإنسان السلطة التنفيذية الرئيس محمود عباس مجلس الوزراء الفلسطيني رئيس الوزراء محمد اشتية السلطة التشريعية المجلس الوطني الفلسطيني المجلس التشريعي الفلسطيني السلطة ال…

Berikut ini adalah daftar tempat di Inggris yang memiliki hubungan dengan komunitas daerah di negara lain. Hubungan ini disebut sebagai kota kembar. Kota Glastonbury beserta nama-nama kota kembarnya Pembagian administratif di Inggris A Abingdon-on-Thames[1] Argentan, Prancis Lucca, Italia Schongau, Jerman Sint-Niklaas, Belgia Adur[2] Riom, Prancis Żywiec, Polandia Alnwick[3] Lagny-sur-Marne, Prancis Time, Norwegia Voerde, Jerman Alton[4] Montecchio Maggiore, Ital…

Deliveroo Holdings plcJenisPerusahaan publikKode emitenLSE: ROOIndustriPemesanan makanan daringPengiriman makananDidirikan2013; 11 tahun lalu (2013)PendiriWill ShuKantorpusatLondon, Inggris, Britania RayaWilayah operasiBritania RayaBelandaPrancisBelgiaIrlandiaItaliaAustraliaSingapuraHong KongKuwaitUni Emirat ArabTokohkunciWill Shu (CEO) Greg Orlowski Dan Winn (CTO) Rohan Pradhan (COO)Pendapatan£476 juta (2018) [1]KaryawanSekitar 2.300 (2020) Sekitar 30.000 kurir lepas (hingga Septe…

Pour les articles homonymes, voir Télévision par câble (South Park). Le câble coaxial est le plus souvent exploité pour distribuer le signal de télévision par câble. La télévision par câble ou télédistribution par câble désigne un mode de distribution de programmes de télévision et accessoirement, de radio, véhiculé par l'intermédiaire d'un réseau câblé, par liaison de type coaxiale ou fibre optique. À de rares exceptions pour certains pays comme la Suisse, l'offre télé…

Si ce bandeau n'est plus pertinent, retirez-le. Cliquez ici pour en savoir plus. Cet article ne cite pas suffisamment ses sources (janvier 2022). Si vous disposez d'ouvrages ou d'articles de référence ou si vous connaissez des sites web de qualité traitant du thème abordé ici, merci de compléter l'article en donnant les références utiles à sa vérifiabilité et en les liant à la section « Notes et références ». En pratique : Quelles sources sont attendues ? Comm…

Moldovan footballer Eugeniu Cociuc Cociuc with Moldova in 2015Personal informationFull name Eugeniu CociucDate of birth (1993-05-11) 11 May 1993 (age 30)Place of birth Chișinău, MoldovaHeight 1.80 m (5 ft 11 in)Position(s) MidfielderTeam informationCurrent team PyunikYouth career Dacia Buiucani 2Senior career*Years Team Apps (Gls)2012–2016 Dacia Chișinău 75 (6)2016–2018 Žilina 17 (2)2018 → Sabail (loan) 13 (2)2018–2019 Sabail 25 (5)2020 Sabah 12 (0)2021 Keşla 5 …

Historic house in New York, United States United States historic placeLevi Ball HouseU.S. National Register of Historic Places Show map of New YorkShow map of the United StatesLocationNY 38,Berkshire, New YorkCoordinates42°19′31″N 76°10′56″W / 42.32528°N 76.18222°W / 42.32528; -76.18222Area12 acres (4.9 ha)Built1840Architectural styleGreek Revival, FederalMPSBerkshire MRANRHP reference No.84003075[1]Added to NRHPJuly 2, 1984 The Lev…

Questa voce sull'argomento società calcistiche cipriote è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. A.S. Othellos AthīainouCalcio Segni distintivi Uniformi di gara Casa Trasferta Colori sociali Verde, bianco Dati societari Città Athīenou Nazione Cipro Confederazione UEFA Federazione CFA Campionato A' Katīgoria Fondazione 1933 Presidente Vassilis Kafataris Allenatore Alexandros Garpozīs Stadio Stadio Othellos Athienou, Athienou(5.000 posti)…

Radio station in Kitchener, Ontario, Canada CJDV-FMKitchener, OntarioBroadcast areaWaterloo RegionFrequency107.5 MHz (FM)Branding107.5 Dave RocksProgrammingFormatActive rockOwnershipOwnerCorus Entertainment(591589 B.C. Ltd.)Sister stationsCKBT-FMHistoryFirst air date1954 (AM)1998 (92.9 FM)2003 (107.5 FM)Former call signsCKGR (1954-1956)CFTJ (1956-1987)CIAM (1987-1998)CIZN-FM (1998-2003)Former frequencies1110 kHz (AM) (1954-1975)1320 kHz (1975-1977)960 kHz (1977-1998)92.9 MHz (FM) (1998-2003)Call…

Human settlement in EnglandSelborneGilbert White's house, The WakesSelborneLocation within HampshirePopulation1,288 (2011 Census including Oakhanger)[1]OS grid referenceSU741366Civil parishSelborneDistrictEast HampshireShire countyHampshireRegionSouth EastCountryEnglandSovereign stateUnited KingdomPost townAltonPostcode districtGU34PoliceHampshire and Isle of WightFireHampshire and Isle of WightAmbulanceSouth Central UK ParliamentEast Hampshire List of …
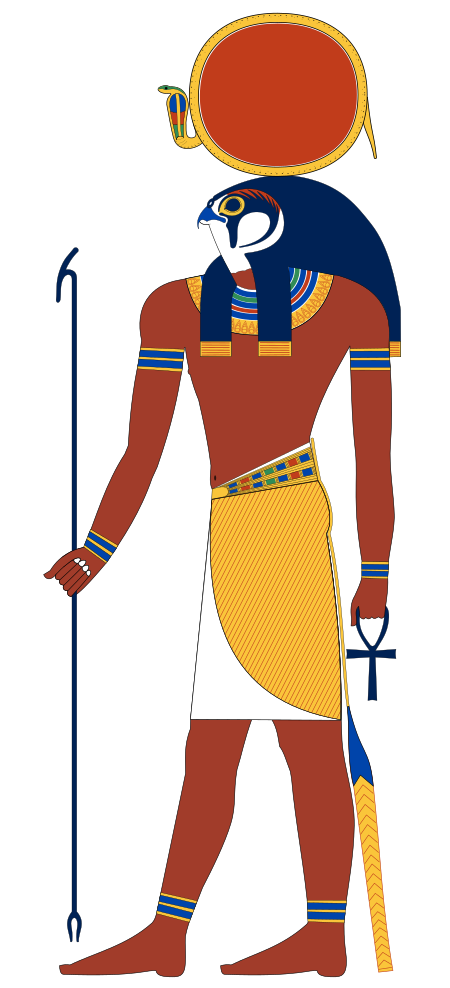
La dea del cielo Nut e le figure delle costellazioni. Tomba di Ramses VI. Ipetueretemkhetnut (La grande Ipet nel grembo di Nut) è una divinità egizia appartenente alla religione dell'antico Egitto, dea-ippopotamo che personificava uno dei dodici mesi dell'anno egizio[1]. Nell'ambito del calendario sotiaco (cioè basato sul ciclo del sistema di Sirio, la stella della costellazione del Cane, dedicata ad Iside e detta Soped dagli Egizi e Sothis dai Greci), Ipetueretemkhetnut rappresentava…

University of Wrocław Botanical GardenBotanic Garden - WroclawTypeBotanical gardenLocationul. Henryka Sienkiewicza 2350-335 WrocławCoordinates51°6′57.01″N 17°2′51.07″EArea7.4 hectares (74,000 m2)Created1811 (1811)Operated byUniversity of WrocławOpenfrom April 5 to October 30WebsiteOfficial webpage (in Polish) The Botanical Garden of the University of Wrocław is a botanical garden in Wrocław founded in 1811 in the area of Ostrów Tumski. The garden was established fo…

1. SNL 2016-2017Prva liga Telekom Slovenije 2016./17. Competizione Campionato sloveno Sport Calcio Edizione 26ª Organizzatore NZS Date dal 16 luglio 2016al 3 giugno 2017 Luogo Slovenia Partecipanti 10 Risultati Vincitore Maribor(14º titolo) Retrocessioni RadomljeKoper Statistiche Miglior giocatore Dare Vršič[1] Miglior marcatore John Mary (17 reti) Miglior portiere Jasmin Handanovič Incontri disputati 180 Gol segnati 463 (2,57 per incontro) Pubblico…

Arrondissement de Château-Salins Situation de l'arrondissement de Château-Salins dans le département Moselle. Administration Pays France Région Lorraine Département et collectivité territoriale Moselle Chef-lieu Château-Salins Code arrondissement 57 2 Démographie Population 29 867 hab. (2011) Densité 31 hab./km2 Géographie Coordonnées 49° nord, 7° est Superficie 974 km2 Subdivisions Cantons 5 Communes 128 modifier L'arrondissement de Château-Sali…

Fictional character from the television series Steven Universe Fictional character Steven UniverseSteven Universe characterLeft: Steven's appearance in Steven UniverseRight: Steven’s appearance in Steven Universe FutureFirst appearanceThe Time Thing (2013) (pilot debut)Gem Glow (2013) (proper series debut)Last appearanceThe Future (2020)Created byRebecca SugarVoiced byZach CallisonDaniel DiVenere (MultiVersus)[1]In-universe informationFull nameSteven Quartz UniverseSpeciesHuman-Gem hyb…

土库曼斯坦总统土库曼斯坦国徽土库曼斯坦总统旗現任谢尔达尔·别尔德穆哈梅多夫自2022年3月19日官邸阿什哈巴德总统府(Oguzkhan Presidential Palace)機關所在地阿什哈巴德任命者直接选举任期7年,可连选连任首任萨帕尔穆拉特·尼亚佐夫设立1991年10月27日 土库曼斯坦土库曼斯坦政府与政治 国家政府 土库曼斯坦宪法 国旗 国徽 国歌 立法機關(英语:National Council of Turkmenistan) 土�…

American public servant and naturalist (1965–2023) Buzzy PeltolaPeltola in 2018Regional Director for Alaska for the Bureau of Indian AffairsIn officeJuly 9, 2018 – July 29, 2022Member of the Bethel City CouncilIn officeOctober 2011 – October 2013 Personal detailsBornEugene R. Peltola Jr.(1966-02-07)February 7, 1966Bethel, Alaska, U.S.DiedSeptember 12, 2023(2023-09-12) (aged 57)Alaska, U.S.Cause of deathAviation accidentCitizenshipUnited StatesOrutsararmiut …

