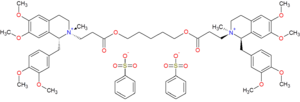
Cisatracurium besilate
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Charles V yang BijaksanaRaja PrancisBerkuasa8 April 1364 – 16 September 1380Prancis19 Mei 1364PendahuluJohn IIPenerusCharles VIInformasi pribadiKelahiran(1338-01-21)21 Januari 1338Vincennes, PrancisKematian16 September 1380(1380-09-16) (umur 42)Beauté-sur-Marne, PrancisPemakamanBasilika Santo DenisWangsaWangsa ValoisAyahJohn II dari PrancisIbuBonne dari BohemiaPasanganJoanna of BourbonAnakCharles VI dari PrancisLouis I, Duke of OrléansCatherine of ValoisAgamaKatolik Roma Charles V (21 J…

Artikel ini sebatang kara, artinya tidak ada artikel lain yang memiliki pranala balik ke halaman ini.Bantulah menambah pranala ke artikel ini dari artikel yang berhubungan atau coba peralatan pencari pranala.Tag ini diberikan pada Oktober 2016. Kurzweil Music Systems adalah sebuah perusahaan Amerika Serikat yang memproduksi elektronik instrumen-instrumen musik untuk pengguna profesional dan nonprofesional. Didirikan pada tahun 1982 oleh Stevie Wonder, Raymond Kurzweil, seorang pengembang dari me…

Penghargaan Bravo adalah penghargaan tahunan yang diberikan oleh Guerin Sportivo, majalah sepak bola Italia ke pemain sepak bola muda terbaik di Eropa. Digelar sejak 1978, penghargaan ini pertama kali dimenangi oleh Jimmy Case. Pemenang Berkas:Jimmy Case cropped.jpgJimmy Case, pemenang pada tahun 1978 Berkas:Ryan Giggs 2014 cropped.jpgRyan Giggs, pemenang pada tahun 1993 Alessandro Del Piero, pemenang pada tahun 1996 Berkas:Lionel Messi cropped.jpgLionel Messi, pemenang pada tahun 2007 Tahun Pem…

Tetrafenilmetana Nama Nama IUPAC (preferensi) 1,1′,1′′,1′′′-Metanatetrailtetrabenzena Penanda Nomor CAS 630-76-2 Y Model 3D (JSmol) Gambar interaktif 3DMet {{{3DMet}}} ChemSpider 11917 Y Nomor EC PubChem CID 12424 Nomor RTECS {{{value}}} UNII CM2Q9C446P Y CompTox Dashboard (EPA) DTXSID30212215 InChI InChI=1S/C25H20/c1-5-13-21(14-6-1)25(22-15-7-2-8-16-22,23-17-9-3-10-18-23)24-19-11-4-12-20-24/h1-20H YKey: PEQHIRFAKIASBK-UHFFFAOYSA-N YInChI=1/C25H20/c1-5-1…
كلاوس بو لارسن معلومات شخصية الميلاد 28 أكتوبر 1965 (العمر 58 سنة)أودنسه الجنسية مملكة الدنمارك تعديل مصدري - تعديل كلاوس بو لارسين (بالدنماركية: Claus Bo Larsen) (مواليد 28 أكتوبر 1965 في أودنسه) حكم كرة قدم دنماركي .[1] قرر الاتحاد الدولي لكرة القدم اعتماده حكما دوليا في سنة 1996 .…

AscoliNama lengkapAscoli Calcio 1898 SpAJulukanPicchio (Burung Pelatuk), Bianconeri (Putih-Hitam)Berdiri1898StadionStadio Cino e Lillo Del Duca,Ascoli Piceno, Italia(Kapasitas: 20.000)Ketua Roberto BenigniHead Coach GustinettiLigaSerie B2022-23ke-12 Kostum kandang Kostum tandang Ascoli Calcio 1898 adalah sebuah klub sepak bola Italia yang didirikan pada tahun 1898. Bermarkas di Ascoli Piceno, Marche, Ascoli pada musim 2013/14 bermain di Lega Pro Prima Divisione. Klub ini memainkan pertandingan k…

Prasasti yang memperingati kematian seekor lembu Apis pada tahun keenam pemerintahan Bakenranef, ditemukan di Serapeum Saqqarah. Nama Bakenranef terlihat di ujung prasasti. Bakenranef, yang dikenal oleh bangsa Yunani Kuno dengan nama Bocchoris[1] merupakan raja yang memerintah Dinasti kedua puluh empat Mesir secara singkat. Berbasis di Sais, Delta barat, ia bertakhta di Mesir Hilir dari sekitar 725 SM hingga 720 SM. Tetapi sejarawan Mesir période Ptolemaik, Manetho[2] menganggap…

Pemukiman Harrison Barat di County Franklin Lokasi di Vermont County Franklin adalah sebuah county yang terletak di negara bagian Vermont, Amerika Serikat. Pada sensus 2020, jumlah penduduknya adalah 49.946 jiwa.[1] Pusat pemerintahannya adalah kota St. Albans.[2] Berbatasan dengan provinsi Quebec di Kanada. County ini dibentuk pada tahun 1792 dan diorganisasi pada tahun 1796.[3][4] County Franklin merupakan bagian dari wilayah metropolitan Burlington. Sejarah Cou…

Gamera ResurgencePoster teaser dengan garis tagar yang diterjemahkan sebagai Jepang vs. Godzilla[1]Nama lainJepangシン・ゴジラHepburnShin Gojira SutradaraHideaki AnnoShinji HiguchiProduser Minami Ichikawa Taichi Ueda SkenarioHideaki AnnoPemeran Hiroki Hasegawa Yutaka Takenouchi Satomi Ishihara Penata musikShirō Sagisu[2][3]Perusahaanproduksi Toho Pictures Cine Bazar DistributorTohoTanggal rilis 29 Juli 2016 (2016-07-29) NegaraJepangBahasaJepangIngg…

2002 single by PulpBad Cover VersionSingle by Pulpfrom the album We Love Life Released15 April 2002Recorded2000–01GenreBritpopLength4:10 / 3:58LabelIslandSongwriter(s)Nick Banks, Jarvis Cocker, Candida Doyle, Steve Mackey and Mark WebberProducer(s)Scott Walker, Chris ThomasPulp singles chronology Sunrise/The Trees (2001) Bad Cover Version (2002) After You (2013) Bad Cover Version is a song by British rock band Pulp, from their 2001 album We Love Life. It was released 15 April 2002 as the secon…

Peta infrastruktur dan tata guna lahan di Komune Bertrimoutier. = Kawasan perkotaan = Lahan subur = Padang rumput = Lahan pertanaman campuran = Hutan = Vegetasi perdu = Lahan basah = Anak sungaiBertrimoutier merupakan sebuah komune di departemen Vosges yang terletak pada sebelah timur laut Prancis. Lihat pula Komune di departemen Vosges Referensi INSEE lbsKomune di departemen Vosges Les Ableuvenettes Ahéville Aingeville Ainvelle Allarmont Ambacour…

威廉·莱昂·麦肯齐·金阁下The Rt Hon. William Lyon Mackenzie KingOM CMG PC 加拿大总理任期1921年12月29日—1926年6月28日君主乔治五世前任阿瑟·米恩继任阿瑟·米恩任期1926年9月25日—1930年8月7日君主乔治五世前任阿瑟·米恩继任理查德·贝德福德·贝内特任期1935年10月23日—1948年11月15日君主乔治五世爱德华八世乔治六世前任理查德·贝德福德·贝内特继任路易·圣洛朗 个人资料出生(187…

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁地�…

Medieval Berber tribal confederation This article may require copy editing for grammar, style, cohesion, tone, or spelling. You can assist by editing it. (May 2024) (Learn how and when to remove this message) Distribution of Berber-speaking groups today. The pink areas depict Western Berber languages: Zenaga to the West, Mauritania and Senegal; Tetserret to the East, Niger. The Sanhaja (Arabic: صنهاجة, Ṣanhaja or زناگة Znaga; Berber languages: Aẓnag, pl. Iẓnagen, and also Aẓna…

German-born opera soprano (1915–2006) DameElisabeth SchwarzkopfDBEThe soprano around 1950BornOlga Maria Elisabeth Friederike Schwarzkopf(1915-12-09)9 December 1915Jarotschin, Kingdom of Prussia, German Empire (now Poland)Died3 August 2006(2006-08-03) (aged 90)Schruns, Vorarlberg, AustriaCitizenship Germany Austria United Kingdom Occupations Classical soprano Voice teacher Organizations Deutsche Oper Berlin Vienna State Opera Salzburg Festival Title Kammersängerin Honorary member of the R…
Logo NET. Berikut ini adalah daftar penyiar NET. Penyiar saat ini Khusus Fakta dan Jatanras Adam Suryanagara Aiptu Zakaria Jacklyn Choppers Ajeng Suseno Angie Ang (mantan penyiar Kompas TV Jawa Tengah) Anjana Demira Devina Bertha Indah Setyani Maria Anatasya (mantan penyiar iNews Semarang) Vannico Soekarno (mantan penyiar Trans TV) Non Berita (Hiburan) Program Televisi Tonight Show Vincent Rompies Deddy Mahendra Desta Hesti Purwadinata Enzy Storia Tonight Festival Vincent Rompies Deddy Mahendra …

Disambiguazione – Se stai cercando il giocatore di snooker inglese, vedi Robbie Williams (giocatore di snooker). Robbie WilliamsRobbie Williams durante la cerimonia di apertura del campionato mondiale di calcio 2018 Nazionalità Regno Unito GenerePop[1]Britpop[1] Periodo di attività musicale1990 – in attività (dal 1996 come solista) GruppiTake That Album pubblicati20 Studio12 Live1 Raccolte7 Sito ufficiale Modifica dati su Wikidata · Manuale Ro…

San Jose Earthquakes 2012 soccer seasonSan Jose Earthquakes2012 seasonOwnerEarthquakes Soccer, LLCCoachFrank YallopStadiumBuck Shaw StadiumMajor League SoccerConference: 1stOverall: 1stMLS Cup PlayoffsConference SemifinalsU.S. Open CupQuarterfinalsCalifornia Clásico1st (2–0–1)Heritage Cup1st (2–0–0)Timbers Tournament1st (1–0–2)Top goalscorerLeague: Chris Wondolowski (27)All: Chris Wondolowski (32)Highest home attendance50,391 v Los Angeles Galaxy (June 30, 2012)Lowest home attendanc…

English footballer Liam Darville Darville playing for York City in 2011Personal informationFull name Liam Thomas Darville[1]Date of birth (1990-10-26) 26 October 1990 (age 33)[1]Place of birth Leyburn, EnglandPosition(s) DefenderTeam informationCurrent team Richmond TownYouth career0000–2010 Leeds UnitedSenior career*Years Team Apps (Gls)2010–2011 Leeds United 0 (0)2010 → Rotherham United (loan) 0 (0)2010–2011 → Tranmere Rovers (loan) 9 (0)2011 York City 17 (0)2011…

هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (ديسمبر 2016) ISOHDFS 27 صورة من مرصد هابل الفضائي للمجرة ISOHDFS 27 مراقبة البيانات (حقبة (فلك) حقبة) الكوكبة الطوقان رمز الفهرس [RFR2001] HDFS 1-406 (A K-band-selected photometric redshift catalog in the Hubble Deep Field So…