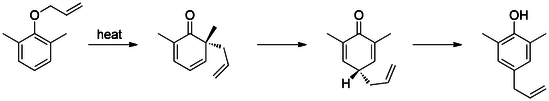
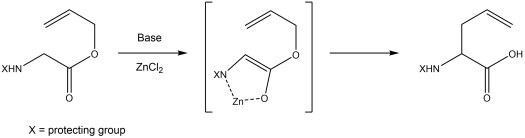
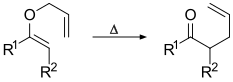Claisen rearrangement
| |||||||||||||||
Read other articles:

Michel Ney, Marsekal Napoleon. Michel Ney, Adipati Elchingen, Pangeran Moskow (10 Januari 1769 – 7 Desember 1815), terkenal dikalangan anak buahnya dengan sebutan Le Rougeaud (si muka merah) dan le Brave des Braves (paling berani di antara pemberani) adalah seorang tentara Prancis dan komandan militer selama Perang Revolusi Prancis dan Perang Napoleon. Dia adalah salah satu dari 26 marsekal Napoleon. Lihat pula Daftar Marsekal Kekaisaran Prancis Pertama Peperangan era Napoleon Catatan Referens…

Monument erected to Ciaran Fleming. Derry Brigade Memorial, Bogside, Derry, August 2009 Kieran or Ciarán Fleming (born 25 October 1959 – 2 December 1984), was a volunteer in the 4th Battalion, Derry Brigade of the Provisional Irish Republican Army (IRA) from the Waterside area of Derry, Northern Ireland.[1] He died while attempting to escape after a confrontation with British troops in 1984.[2][3][4] Background Fleming was the youngest son of Paddy and Maud…

Pewarta SoerabaiaPewarta SoerabaiaTipeHarianPemilikThe Kian SingPendiriH.KommerPemimpin redaksiCourant dan H.KommerBahasaBahasa MelayuPusatKota Soerabaia Pewarta Soerabaia adalah sebuah surat kabar Melayu-Tionghoa yang pernah ada di Indonesia, diterbitkan di Surabaya. Surat kabar ini didirikan pada tahun 1902 oleh Kommer, seorang warga Belanda. Artikel bertopik jurnalisme ini adalah sebuah rintisan. Anda dapat membantu Wikipedia dengan mengembangkannya.lbs

This is an incomplete list that refers to those who were not from the Ottoman Empire, but later served the country. This may be militarily, as a diplomat, a spy, or any other way. Foreigners employed by the Sublime Porte, often renegades and refugees, were diverse in their ethnic origins, generally hailing from aristocratic families. Typically high-ranking individuals in Ottoman society, they were regarded as invaluable by the Sultan, and were therefore generously rewarded for their services. …

Artikel ini perlu diwikifikasi agar memenuhi standar kualitas Wikipedia. Anda dapat memberikan bantuan berupa penambahan pranala dalam, atau dengan merapikan tata letak dari artikel ini. Untuk keterangan lebih lanjut, klik [tampil] di bagian kanan. Mengganti markah HTML dengan markah wiki bila dimungkinkan. Tambahkan pranala wiki. Bila dirasa perlu, buatlah pautan ke artikel wiki lainnya dengan cara menambahkan [[ dan ]] pada kata yang bersangkutan (lihat WP:LINK untuk keterangan lebih lanjut). …

Vicky KaleaLahirVicky Hidayat19 Juli 1993 (umur 30)[1][2]Cirebon, Jawa Barat, IndonesiaPendidikanSMA Negeri 1 LemahabangPekerjaanPemeranpenyanyimodelTahun aktif2016—sekarangSuami/istriAlliza Putri Qistifany (m. 2022)Karier musikInstrumenVokal Vicky Hidayat (lahir 19 Juli 1993), lebih dikenal sebagai Vicky Kalea adalah pemeran dan penyanyi Indonesia. Kehidupan awal Vicky lahir dengan nama Vicky Hidayat pada 19 Juli 1993 di Kota Ci…

Polo air pada Pekan Olahraga Nasional 2021LokasiArena Akuatik, Kawasan Olahraga Kampung Harapan, Kabupaten JayapuraTanggal23 September–3 Oktober 2021PesertaTBD← 20162024 → Polo air pada Pekan Olahraga Nasional 2021 akan berlangsung di Kabupaten Jayapura dan menandingkan baik nomor putra maupun putri. Kualifikasi Tim putra Jalur Kualifikasi Lokasi Provinsi yang Lolos Tuan rumah — Papua Indonesia Open Aquatics Championship Jakarta Daerah Khusus Ibukota Jakarta…

Distrik Bisnis Wuhan武汉商务区LokasiDistrik Jianghan, Wuhan, HubeiTiongkokOperatorWuhan Metro Co., LtdJumlah peron2 (1 peron pulau)KonstruksiJenis strukturBawah tanahSejarahDibuka28 Desember 2015 (Jalur 3)Sunting kotak info • L • BBantuan penggunaan templat ini Stasiun Distrik Bisnis Wuhan (Hanzi: 武汉商务区站), adalah stasiun Jalur 3 dan Jalur 7 Wuhan Metro, yang mulai beroperasi pada 28 Desember 2015. Stasiun ini terletak di Distrik Jianghan, sesuai namanya stasiun…

Alcatraz IslandPoster rilis teatrikalSutradaraWilliam C. McGannProduserBryan FoySkenarioCrane WilburPemeranJohn LitelAnn SheridanMary MaguireGordon OliverDick PurcellBen WeldenPenata musikHeinz RoemheldSinematograferL. William O'ConnellPenyuntingFrank DeWarPerusahaanproduksiCosmopolitan ProductionsDistributorWarner Bros.Tanggal rilis 6 November 1937 (1937-11-06) Durasi63 menitNegaraAmerika SerikatBahasaInggris Alcatraz Island adalah film Amerika Serikat produksi tahun 1937 bergenre dr…

Species of lizard Leaden delma Conservation status Least Concern (IUCN 3.1)[1] Scientific classification Domain: Eukaryota Kingdom: Animalia Phylum: Chordata Class: Reptilia Order: Squamata Family: Pygopodidae Genus: Delma Species: D. plebeia Binomial name Delma plebeiaDe Vis, 1888 The leaden delma (Delma plebeia) is a species of lizard in the Pygopodidae family endemic to Australia.[2] References ^ Wilson, S.; Hobson, R.; Vanderduys, E.; Venz, M.; Dickman, C. (2018). …

Chapter of the New Testament Revelation 12← chapter 11chapter 13 →Apocalypse 19. Michael and the angel. Revelation 12:7-9. Scheits. Phillip Medhurst CollectionBookBook of RevelationCategoryApocalypseChristian Bible partNew TestamentOrder in the Christian part27 Revelation 12 is the twelfth chapter of the Book of Revelation or the Apocalypse of John in the New Testament of the Christian Bible. The book is traditionally attributed to John the Apostle,[1][2] but the pr…

Bernd DittertBernd Dittert lors du championnat de RDA de poursuite à Cottbus en 1988.InformationsNaissance 6 février 1961 (63 ans)GenthinNationalité allemandeDistinctions Médaille d'or de l'ordre du Mérite patriotiqueÉtoile de l'amitié des peuplesPrincipales victoires Champion olympique du contre-la-montre par équipes (1992) Champion du monde de poursuite par équipes (1981)modifier - modifier le code - modifier Wikidata Bernd Dittert, né le 6 février 1961 à Genthin, est un coure…

Rearrangement of sectors on a hard disk into contiguous units Defrag redirects here. For other uses, see Defrag (disambiguation). Disk Defragmenter redirects here. For the Microsoft Windows utility, see Microsoft Drive Optimizer. Visualization of fragmentation and then of defragmentation In the maintenance of file systems, defragmentation is a process that reduces the degree of fragmentation. It does this by physically organizing the contents of the mass storage device used to store files into t…

Fictional character in Sailor Moon The native form of this personal name is Aino Minako. This article uses Western name order when mentioning individuals. Fictional character Sailor VenusPretty Soldier Sailor Moon and Codename: Sailor V characterMinako in her Super Sailor Venus form as seen in Season 4 of the original anime.First appearanceCodename: Sailor V chapter #1: Sailor V is Born! (1991)Created byNaoko TakeuchiVoiced byJapanese: Rika Fukami Shizuka Itō (Sailor Moon Crystal) English: …
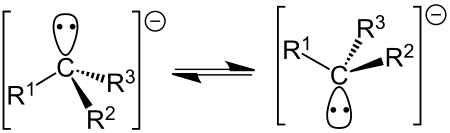
Karbanion Karbanion adalah sejenis anion dari karbon yang memiliki satu pasangan elektron menyendiri. Karbanion memiliki geometri trigonal piramida dan secara formal merupakan konjugat basa dari asam karbon: R3C-H + B− → R3C− + H-B dengan B merujuk pada basa. Karbanion merupakan salah satu dari beberapa zat antara reaktif kimia organik. Teori Karbanion merupakan sejenis nukleofil. Stabilitas dan reaktivitas karbanion ditentukan oleh beberapa faktor, yaitu: Efek induktif. Atom-atom elektron…

Italian cyclist Angelo GremoGremo in 1922Personal informationFull nameAngelo GremoBorn3 December 1887Turin, ItalyDied4 September 1940(1940-09-04) (aged 52)Turin, ItalyTeam informationDisciplineRoadRoleRiderProfessional teams1911Fiat1912–1913Peugeot1913Griffon-Continental1914Peugeot1914Automoto1914Ganna-dunlop1915Bianchi1916Maino1917Bianchi1918Dei1919Stucchi - Dunlop1920–1922Bianchi - Pirelli1923–1924Maino1925–1926Meteore - Wolber Major winsGrand Tours Giro d'Italia 2 individual…

Youssef Maleh Nazionalità Italia Marocco (dal 2021) Altezza 179 cm Peso 70 kg Calcio Ruolo Centrocampista Squadra Empoli CarrieraGiovanili 2014-2017 CesenaSquadre di club1 2017-2019 Ravenna30 (1)[1]2019-2021 Venezia57 (5)[2]2021-2023 Fiorentina35 (2)2023 Lecce17 (0)2023-→ Empoli28 (0)Nazionale 2020-2021 Italia U-215 (1)2023- Marocco1 (0) 1 I due numeri indicano le presenze e le reti segnate, per le sole partite di campionato.Il s…

Torpedo bomber and maritime reconnaissance floatplane Do 22 Dornier Do 22 K in the Finnish Air Force Role Torpedo bomber and reconnaissance seaplaneType of aircraft National origin Germany Manufacturer Dornier Flugzeugwerke First flight 15 July 1938 Produced 1938–1939 Number built ~30 The Dornier Do 22 was a German seaplane, developed in the 1930s. Despite good performance, it was built only in small numbers and entirely for the export market. The type was operated in the Second World War by F…

此條目可参照英語維基百科相應條目来扩充。 (2021年5月6日)若您熟悉来源语言和主题,请协助参考外语维基百科扩充条目。请勿直接提交机械翻译,也不要翻译不可靠、低品质内容。依版权协议,译文需在编辑摘要注明来源,或于讨论页顶部标记{{Translated page}}标签。 约翰斯顿环礁Kalama Atoll 美國本土外小島嶼 Johnston Atoll 旗幟颂歌:《星條旗》The Star-Spangled Banner約翰斯頓環礁地�…

「アプリケーション」はこの項目へ転送されています。英語の意味については「wikt:応用」、「wikt:application」をご覧ください。 この記事には複数の問題があります。改善やノートページでの議論にご協力ください。 出典がまったく示されていないか不十分です。内容に関する文献や情報源が必要です。(2018年4月) 古い情報を更新する必要があります。(2021年3月)出典�…