Remimazolam
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Dr. dr.Nova Riyanti YusufSp.KJ Anggota Dewan Perwakilan RakyatRepublik IndonesiaMasa jabatan2 Oktober 2018 – 30 September 2019Pengganti Antar Waktu[1] PendahuluVenna MelindaPenggantiPetahanaDaerah pemilihanJawa Timur VIMasa jabatan1 Oktober 2009 – 30 September 2014Daerah pemilihanDKI Jakarta II Informasi pribadiLahir27 November 1977 (umur 46)Palu, Sulawesi Tengah, IndonesiaKebangsaanIndonesiaPartai politikDemokratAlma materUniversitas TrisaktiUniversitas Indone…
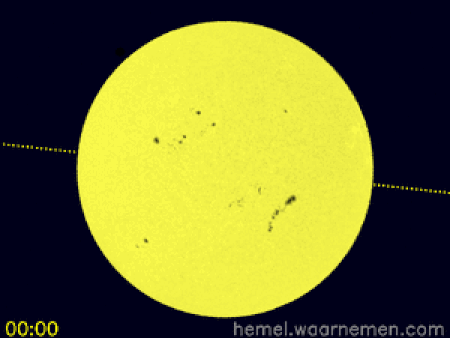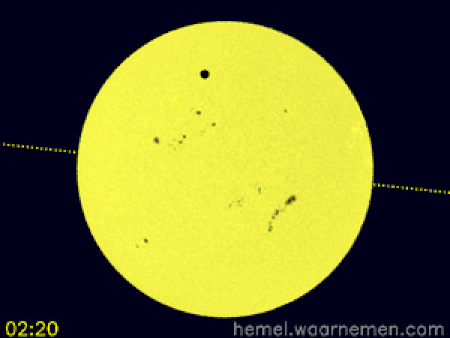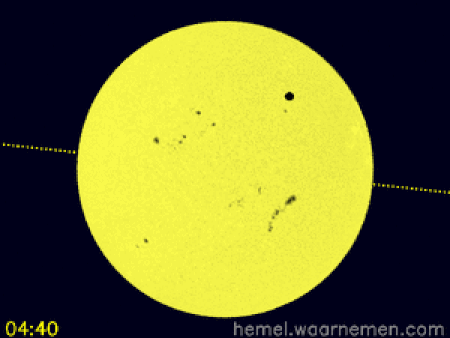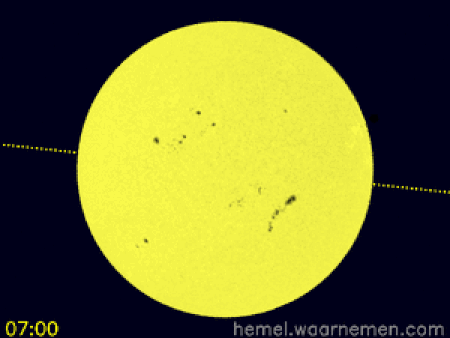
Animasi yang menggambarkan simulasi transit di koordinat khatulistiwa dan CEST[1] Transit Venus 2012 terjadi ketika planet Venus muncul sebagai lingkaran kecil dan gelap bergerak di seluruh permukaan Matahari pada hari Rabu, 6 Juni 2012 dimulai dari 6.09 pagi sampai 12.50 tengah hari waktu Malaysia. Transit Venus adalah fenomena yang langka. Ia pernah terjadi pada 1639, 1761, 1769, 1874, 1882 dan bulan Juni 2004. Fenomena ini hanya terjadi lagi pada Desember 2117 dan Desember 2125. Trans…
Koordinat: 53°49′19″N 1°30′32″W / 53.822°N 1.509°W / 53.822; -1.509 Gledhow Valley Road, persimpangan dengan Gledhow Lane Gledhow adalah daerah suburban di timur laut Leeds, West Yorkshire, Inggris, terletak di sebelah timur Chapel Allerton dan barat Roundhay. Daerah ini secara administratif masuk ke dalam Ward Dewan Kota Roundhay. Ciri utama daerah tersebut adalah Gledhow Valley, sebuah daerah memanjang dari hutan dengan anak sungai kecil dan danau. Sebuah pe…

Giuliano FerraraGiuliano Ferrara nel 1992 Ministro per i rapporti con il ParlamentoDurata mandato11 maggio 1994 –17 gennaio 1995 Capo del governoSilvio Berlusconi PredecessorePaolo Barile SuccessoreGuglielmo Negri EuroparlamentareDurata mandato25 luglio 1989 –11 maggio 1994 LegislaturaIII GruppoparlamentarePSE CircoscrizioneItalia centrale Sito istituzionale Dati generaliPartito politicoPCI (1973-1982)PSI (1985-1994)FI (1994-2008)Aborto? No grazie (2008) Prof…

Asosiasi Sepak Bola Kepulauan FaroeUEFADidirikan1979Kantor pusatTórshavnBergabung dengan FIFA1988Bergabung dengan UEFA1990PresidenChristian AndreasenWebsitehttps://www.fsf.fo Asosiasi Sepak Bola Kepulauan Faroe (bahasa Inggris: Faroe Islands Football Association) (bahasa Faroe: Fótbóltssamband Føroya; FSF) adalah badan pengendali sepak bola di Kepulauan Faroe. Badan ini mengorganisasi Liga Utama Kepulauan Faroe dan tim nasional sepak bola Kepulauan Faroe. FSF berkantor pusat di Tórshavn…

Cet article est une ébauche concernant le modélisme, le maquettisme et les modèles réduits et un bateau ou un navire. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. En 1898, Tesla fait la démonstration d’un bateau radiocommandé (en) Brevet U.S. 613,809 - Méthode d'un appareil de contrôle du mécanisme d'un véhicule ou de véhicules en mouvement. Un bateau radiocommandé est un bateau contrôlé à…

Ligase (Inggris: ligase, synthase, synthetase, carboxylase) merupakan golongan enzim yang berfungsi melepaskan molekul asam pirofosfat dari molekul induknya berupa isomer asam trifosfat (termasuk ATP), sehingga molekul induknya terbelah menjadi dua.[1] Reaksi pelepasan tersebut akan disertai dengan pembentukan molekul baru dan biasanya juga disertai dengan reaksi hidrolisis.[2] Secara umum, reaksi katalisis yang terjadi: Ab + C → A–C + b atau kadang-kadang Ab + cD → A�…

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (نوفمبر 2019) دوري هونغ كونغ لكرة القدم 1989–90 تفاصيل الموسم دوري هونغ كونغ الدرجة الأولى [لغات أخرى] النسخة 4…

Pour les articles homonymes, voir Matz (homonymie). le Matz Source du Matz à Canny-sur-Matz. le Matz sur OpenStreetMap. Caractéristiques Longueur 24,9 km [1] Bassin 188 km2 [1] Bassin collecteur la Seine Nombre de Strahler 3 Organisme gestionnaire Syndicat de la vallée du Matz[2] Régime pluvial océanique Cours Source au lieu-dit le Bouillon · Localisation Canny-sur-Matz · Altitude 68 m · Coordonnées 49° 36′ 18″ N, 2° 47′ 59″ E Confl…

Produzione del caccia Bell P-39Q-30-BE Airacobra a Wheatfield, durante la Seconda guerra mondiale. La produzione di massa (detta anche: a flusso, ripetitiva, in serie o seriale, continua) è la realizzazione di grandi quantità di prodotti standardizzati, spesso compiuta con catene di montaggio o linee a trasferimento. Basata sui principi formulati da Frederick Taylor, sull'utilizzo di parti intercambiabili prodotte con macchine utensili rispettando tolleranze di lavorazione, fu grandemente diff…

Condition characterized by partial or complete absence of pigment in the skin, hair and eyes Medical conditionAlbinismOther namesAchromia, achromasia, achromatosisYoung African boy with albinismPronunciationalbino (UK: /ælˈbiːnoʊ/,[1] or US: /ælˈbaɪnoʊ/)[2] SpecialtyDermatology Albinism is a congenital condition characterized in humans by the partial or complete absence of pigment in the skin, hair and eyes. Albinism is associated with a number of vision defects, suc…

2014 Wyoming Secretary of State election ← 2010 November 4, 2014 (2014-11-04) 2018 → Turnout65.05% 3.13% Nominee Ed Murray Jennifer Young Howard Kit Carson Party Republican Constitution Libertarian Popular vote 119,772 18,918 16,858 Percentage 76.58% 12.10% 10.78% County resultsMurray: 70–80% 80–90% Secretary of State before election Max Maxfield Republican Elected Secretary of State …

Town and municipality in Šumadija and Western Serbia, SerbiaSjenica Сјеница (Serbian)Town and municipalityFrom top: Uvac Special Nature Reserve, Sjenica Mosque at night Coat of armsLocation of the municipality of Sjenica within SerbiaCoordinates: 43°16′N 20°00′E / 43.267°N 20.000°E / 43.267; 20.000Country SerbiaRegionŠumadija and Western SerbiaDistrictZlatiborFirst mentioned1253Settlements101Government • MayorMunib Mujagić (SPP)Area…

La gatta sul tetto che scottaOpera teatrale in tre atti AutoreTennessee Williams Titolo originaleCat on a Hot Tin Roof Lingua originaleInglese Composto nel1954 Prima assoluta24 marzo 1955Morosco Theatre, Broadway (New York) Prima rappresentazione italiana18 gennaio 1958Teatro Manzoni, Milano Premi Premio Pulitzer 1955 a Tennessee Williams Personaggi Brick Pollitt Maggie la gatta, sua moglie Big Daddy, padre di Brick e Gooper Ilda, moglie di Big Daddy, detta Big Mama Gooper Pollitt, fratello di B…

Pour les articles homonymes, voir Siège de Gérone. Siège de Gérone Informations générales Date 6 mai - 12 décembre 1809 Lieu Gérone, Espagne Issue Victoire française Belligérants Empire français Confédération du Rhin Royaume d'Italie Espagne Commandants Laurent de Gouvion-Saint-Cyr puisPierre Augereau Mariano Álvarez de Castro Blacke depuis l'extérieur Forces en présence 35 000 hommes 40 canons 5 600 hommes<Abu Malik Abd al-Wahidbr />200 canons Pert…

American politician (born 1970) Frank GuintaMember of the U.S. House of Representativesfrom New Hampshire's 1st districtIn officeJanuary 3, 2015 – January 3, 2017Preceded byCarol Shea-PorterSucceeded byCarol Shea-PorterIn officeJanuary 3, 2011 – January 3, 2013Preceded byCarol Shea-PorterSucceeded byCarol Shea-Porter54th Mayor of ManchesterIn officeJanuary 3, 2006 – January 3, 2010Preceded byRobert A. BainesSucceeded byTed GatsasCity of Manchester Ald…
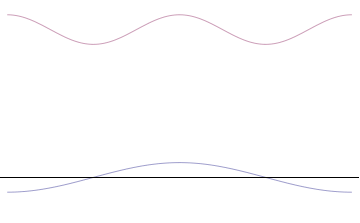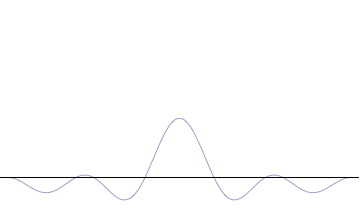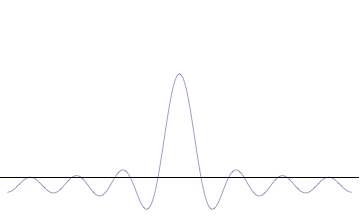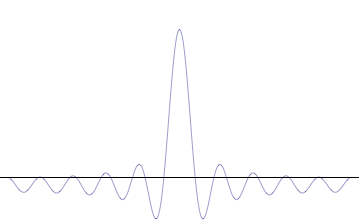
Foundational principle in quantum physics For other uses, see Uncertainty principle (disambiguation). Part of a series of articles aboutQuantum mechanics i ℏ d d t | Ψ ⟩ = H ^ | Ψ ⟩ {\displaystyle i\hbar {\frac {d}{dt}}|\Psi \rangle ={\hat {H}}|\Psi \rangle } Schrödinger equation Introduction Glossary History Background Classical mechanics Old quantum theory Bra–ket notation Hamiltonian Interference Fundamentals Complementarity Decoherence Entanglemen…

English writer and publisher (1873–1939) Ford Madox Fordc. 1905 photoBornJoseph Leopold Ford Hermann Madox Hueffer(1873-12-17)17 December 1873Merton, Surrey, EnglandDied26 June 1939(1939-06-26) (aged 65)Deauville, FrancePen nameFord Madox FordOccupationNovelist, publisherPeriod1873–1939SpouseElsie Martindale HuefferPartnerViolet HuntStella BowenJanice BialaChildren3RelativesFrancis Hueffer (father)Catherine Madox Brown (mother)Oliver Madox Hueffer (brother)Juliet Soskice (sister)F…

Kastil PõltsamaaPõltsamaa linnusPõltsamaa, Estonia Koordinat58°39′19″N 25°58′37″E / 58.655277°N 25.976944°E / 58.655277; 25.976944JenisKastil ordo (reruntuhan)Sejarah situsDibangun1272 (1272)Dibangun olehOrdo Livonia Kastil Põltsamaa Kastil Põltsamaa (bahasa Estonia: Põltsamaa linnus; Jerman: Schloss Oberpahlencode: de is deprecated ), juga disebut Kastil Ordo Põltsamaa, (bahasa Estonia: Põltsamaa ordulinnus), adalah sebuah kastel yan…

Playoff tournament of the NHL 2016 Stanley Cup playoffsTournament detailsDatesApril 13–June 12, 2016Teams16Defending championsChicago BlackhawksFinal positionsChampionsPittsburgh PenguinsRunner-upSan Jose SharksTournament statisticsScoring leader(s)Logan Couture (Sharks) (30 points)MVPSidney Crosby (Penguins)← 20152017 → The 2016 Stanley Cup playoffs was the playoff tournament of the National Hockey League (NHL) for the 2015–16 season. They began on April 13, 2016, and …
